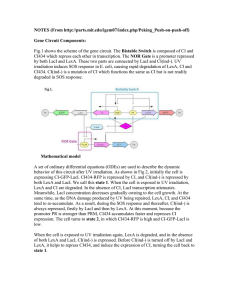
Purification of LexA
advertisement

Purification of LexA 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. Inoculate BL21(DE3) pET28a-His-LexA for O/N @ 37oC Dilute 1:1000 into 500 ml ZYM-5052 media + 100 Kn & grow @ 37 oC 1.5 hrs/250 rpm Shift to room temp & grow O/N (~24 oC for ~14 hrs) Cent in weighed 250 ml PP bottles at 8K/10’, discard supe and weigh pellet. Freeze pellet @ -80 oC 1 hr-O/N Transfer 2 ml of 50% slurry Ni-NTA beads to 5ml column & wash w/ 10 CV Wash buffer. Add Calbiochem Protease inhibitor VII to pellet 100 λ/g Resuspend cells with at least 5 ml BugBuster / g pellet and transfer to 30 ml PP cent tube Rock slowly at room temperature 10-20 min Centrifuge 16,000 g/ 20 min /4 oC and transfer supernatant to 15 ml tube. Cut end off 1 ml tip and use to transfer washed beads to 15 ml tube. Invert beads and clarified cell lysate at room temperature 1 hr. Pour gels and turn on heat block Transfer beads to column and collect flow through in 15 ml tube 10 CV (10 ml) Wash buffer and collect in 15 ml tube Elute as follows: tube # CV [Imidazole] 1, 2 2 100 mM 3 1 150 mM 4 1 200 mM 5 1 250 mM 6, 7 2 300 mM Samples boiled 5-10’, run 12% gel and Coomassie stain (see template below) as follows: Tube # MW Supe WCL FT W 1 2 3 4 5 6 7 beads 7.5 10 + 3λ 4X 20 20 20 20 20 20 20 20 20 20 +6 +6 +6 +6 +6 +6 +6 +6 +6 +6 17. 18. 19. 20. 21. Pool LexA containing fractions with <90% purity and combine with fresh, washed Ni-TA beads in 50 ml tube with 30 ml wash buffer overnight at 4 oC. Repeat steps 13-16 Strip dialysis tubing with 1 mM EDTA and then rinse with water. Dialyze against 1 L of LexA storage buffer 3X, 3 hrs - O/N each time Nanodrop (User: Lichtarge PW: t907) to determine OD280 and use = 7300 M-1 cm-1 with c = Abs / ( * l); LexA MW = 24389.8 g/mol ZYM-5052: 1% Tryptone, 0.5% Yeast extract, 25 mM Na2HPO4, 25 mM KH2PO4, 50 mM NH4Cl, 5 mM Na2SO4, 2 mM MgSO4, 0.5% glycerol, 0.05% glucose, 0.2% α-lactose ZY media 50X M 50X 5052 1M MgSO4 25 mg/ml Kn 190 ml 4 ml 4 ml 400 λ 800 λ ZY media: 1 % Tryptone, 0.5% Yeast extract Tryptone 2g 1g Yeast extract 1 g 0.5 g q.s. H2O 190 ml 80 ml Autoclave 50X M: 1.25M Na2HPO4, 1.25 M KH2PO4, 2.5 M NH4Cl, 250 mM Na2SO4 Na2HPO4 83.8 g KH2PO4 42.5 g NH4Cl 33.4 g Na2SO4 8.88 g q.s. H2O 250 ml filter sterilize 50X 5052: 25% glycerol, 2.5% glucose, 10% α-lactose Glycerol 62.5 ml Glucose 6.25 g α-lactose 25 g q.s. H2O 250 ml filter sterilize Wash buffer: 50 mM PO4, 50 mM Imidazole, 1% glycerol, 500 mM NaCl LexA storage buffer: 10 mM PIPES-NaOH pH 7.0, 0.1 mM EDTA, 200 mM NaCl, 10% glycerol PIPES 9.1 g 0.5 M EDTA 600 λ NaCl 35 g Glycerol 300 ml q.s. H2O 3L pH 7.0 ~ 9 pellets NaOH & 10N NaOH dropwise if necessary Notes: If column gets clogged, can quick spin at 250 g but don’t allow bed to go dry. K156A mutant elution precipitates on ice at high concentrations. Do not leave protein elutions in >50 mM imidazole overnight. From Movahedzadeh, Davis Micro97) This clone was transformed into E. coli BL21(DE3)pLysS. Cultures were grown in LB broth containing 200 lg ampicillin ml−" and 34 lg chloramphenicol ml−", and were induced at OD'!! 0±5 (1 cm pathlength, Unicam UV2 spectrometer) by the addition of IPTG to a ®nal concentration of 0±1 mM (along with more ampicillin to 200 lg ml−") and incubation continued for a further 3 h before harvesting. Purification of M. tuberculosis LexA. The induced cells were harvested, resuspended in sonication buffer (50 mM phosphate pH 8, 1 M NaCl), and lysed by sonication at 100 W for 4¬30 s. The LexA protein was puri®ed by means of the His tag introduced from the vector on a NiNTA (Qiagen) column. The lysate was applied at 1 ml min−", the column washed with 50 mM phosphate pH 5, 1 mM imidazole, 10% (w}v) glycerol, and the LexA protein eluted with 50 mM phosphate pH 8, 1 M NaCl, 0±25 M imidazole. The puri®ed protein was estimated by SDS-PAGE to be "95% pure. Autoinduction media from Studier ProtExpPur05