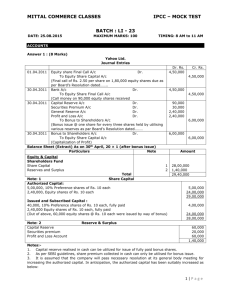
____ Ca(OH)2(aq) + ____ H2(g) → _____ H2O(l) + _____ Ca(s)
advertisement

2/18/2009 Science 20 Unit A: Chemistry Balancing Single Replacement Reactions & Simple Stoich Assignment Part A) Balance each single replacement reaction. (1 mark each) 1) ____ Ca(OH)2(aq) + ____ H2(g) _____ H2O(l) + _____ Ca(s) 2) ____ Zn(s) + ____ H2CO3(aq) _____ H2(g) + _____ ZnCO3(aq) 3) ____ Pb(s) + ____ Hg2S(aq) ______ Hg(s) + ______ PbS(aq) 4) _____ Na(s) + _____ HOH(l) _____ NaOH(aq) + _____ H2(g) 5) _____ Fe(s) + _____ Al2O3(s) _____ Al(s) + _____ Fe2O3(s) LD Industries Name: ___________ Date: ___________ 2/18/2009 Part B) Determine the number of moles required or given using Make sure you show the starting formula, units and proper sig digs to get full marks. 1) Using the equation below, determine the amount of silver (Ag(s)) required to make 0.876 mol of silver sulfide (Ag2S(s)). (2 marks) 4 Ag(s) + 2 H2S(g) + O2(g) 2 Ag2S(s) + 2H2O(l)_ 2) Using the equation below, determine the amount of silver (Ag(s)) produced if 1.5 x 103 mol of iron (III) oxide (Fe2O3(s)) is reacted. (2 marks) Fe2O3(s) + 3 CO(g) 2 Fe(s) + 3 CO2(g) 3) Using the equation below, determine the amount of copper (Cu(l)) produced if 200 mol of copper (I) oxide (Cu2O(l)) is reacted. (2 marks) 2 Cu2O(l) + Cu2S(l) 6 Cu(l) + SO2(g) Bonus Question: Answer Only ONE!!! Fair Bonus Question: Give an example of an ion or atom that can be both reduced and oxidized. Unfair Bonus Question: What is another mnemonic for remembering that Oxidation Is Loss of electrons and Reduction Is Gain of electrons? LD Industries
















![[SCH4U] CHEMISTRY FINAL EXAMINATION](http://s3.studylib.net/store/data/008662124_1-d6e01d8a2ab2169d5499c2d8dca8a7af-300x300.png)