One way to determine the concentration of chloride ions in aqueous
advertisement

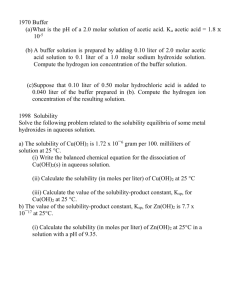
WOLPA/AP CHEMISTRY CHAPTER NINETEEN: PRINCIPLES OF REACTIVITY: PRECIPITATION REACTIONS Problem Set 2 1. The solubility of iron (II) hydroxide, Fe(OH)2 is 1.43 x 10-3 grams per liter at 25 oC. a. Write the balanced equation for the solubility equilibrium b. Write the expression for the solubility product constant, Ksp, and calculate its value. c. Calculate the pH of a saturated solution of Fe(OH)2 at 25 oC. d. A 50.0 milliliter sample of 3.00 x 10-3 M FeSO4 solution is added to 50.0 milliliters of 4.00 x 10-6 molar NaOH solution, Does a precipitate of Fe(OH)2? Explain and show calculations to support your answer. 2. A saturated solution of lead iodate in pure water has a lead ion concentration of 4.0 x 10 -5 mole per liter at 20oC (a) Calculate the value for the solubility product constant of Pb(IO3)2 at 25oC (b) Calculate the molar solubility of Pb(IO3)2 in a 0.10 molar Pb(NO3)2 solution at 25oC (c) To 333 milliliters of a 0.120-molar Pb(NO3)2 solution, 667 milliliters of 0.435molar KlO3 is added. Calculate the concentrations of Pb2+ and IO3- in the solution at equilibrium at 25oC 3. The solubility of Zn(OH)2 is not the same in the following solutions as it is in pure water. In each case state whether the solubility is greater or less than that in water and briefly account for the change in solubility. (a) 1-molar HCI (b) 1-molar Zn(NO3)2 (c) 1-molar NaOH (d) 1-molar NH3 4. H2S + H2O <--------------> H3O+ + HS- K1 = 1.0 x 10-7 HS- + H2O <-----------------> H3O+ + S2- K2= 1.3 x 10-13 H2S + 2 H2O <------------------> 2 H3O+ + S2Ag2S(s) <--------------------> 2 Ag+ + S2- K= 1.3 x 10-20 Ksp= 4.4 x 10-51 (a) Calculate the concentration of H3O+ of a solution which is 0.10 molar in H2S. (b) Calculate the concentration of the sulfide ion, S2-, in a solution that is 0.10 molar in H2S and 0.40 molar in H3O+ (c) Calculate the maximum concentration of silver ion, Ag+, that can exist in a solution that is 1.5 x 10-17 molar in sulfide ion, S2-. 5. How many moles of Ba(IO3)2 is contained in 1.0 liter of a saturated solution of this salt at 25oC? Ksp of Ba(IO3)2 = 6.5 x 10-10 When 0.100 liter of 0.060 molar Ba(NO3)2 and 0.150 liter of 0.12 molar KlO3 are mixed at 25oC, how many milligrams of barium ion remains in each milliliter of the solution? Assume that the volumes are additive. 6. What is the concentration of F- in a saturated solution of BaF2 if Ksp = 1.7 x 10-6? 7. For BaSO4, Ksp = 1.1 x 10-10. What is the solubility of BaSO4, in a solution which is 0.018M in Na2SO4? 8. If 15.0 mg AgCI is placed in 100 ml of water, what is the chloride ion concentration of the solution? Ksp = 1.8 x 10-10. 9. For MgF2, Ksp = 6.4 x 10-9, if you mix 500 ml of 1.0 x 10-4 M Mg( NO3)2 and 500mls of 1.00 x 10-4 M NaF, what will be observed? 10. For CaCrO4, Ksp= 7.1 x 10-4. How many grams of Na2CrO4 must be added to 200mls of 0.250 M Ca(NO3)2 to begin to see a precipitate form?