PHENYTOIN
advertisement

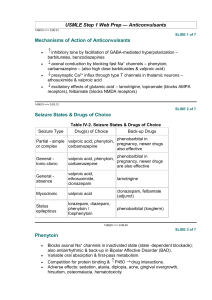
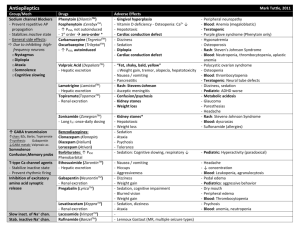
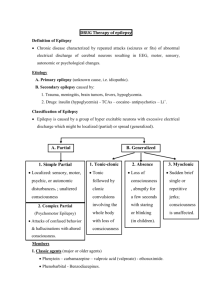
PHENYTOIN PHARMACOKINETIC PROPERTIES Bioavailavility 85 % (70-100%). It is difficult to evaluate because of the drug’s capacitylimited metabolism. ka = 50 mg/h saturable kinetics of absorption (zero order) tmax = 3 - 12 h (dependent of the dose: increase with dose) Vd = 0.65 L/Kg (0.6-0.8) in patients with normal renal function and with normal plasma albumin concentrations. Phenytoin binds primarily to albumin in plasma, being the bound fraction of 0.9 (0.69-0.95) under normal conditions. Protein binding may be greatly reduced in the presence of the following factors: Hypoalbuminaemia - for example, due to severe hepatic or renal disease The last trimester of pregnancy, perhaps because of dilutional hypoalbuminaemia Renal failure because of a reduced affinity of albumin for phenytoin Displacement from protein binding sites by saliylates, valproic acid and sulphonylureas Renal excretion 1-5 % Vmax.C ss Km * D / D / C ss ss Vmax D / Km C Biotransformation 70-90 % (saturable according Michaelis-Menten kinetics). In steady-state: The rate of phenytoin administration must be less than Vmax, otherwise steady-state will never be achieved. Adult patients: Vmax = 6 mg/kg/day (5-15) Km = 5.7 mg/L (1-20) Paediatric patients: Children from 6 months to 6 years Children from 7 to 16 years Children from 6 months to 16 years C.V. = 50 % C.V. = 30 % Vmax = 12 mg/Kg/day (10-13) Vmax = 9 mg/Kg/day (8-20) Km = 4 mg/L (2-13) Autoinduction by phenytoin should be considered during the treatment because enzyme induction can occur after only a brief exposure to the drug. C obs C calcu 0.25. ALB 0.1 Winter-Tozer equation is useful in predicting normalized phenytoin concentrations in patients when unbound phenytoin concentrations are not readily available: In addition, a graphical nomogram method to estimate the phenytoin free concentration at any selected total plasma phenytoin and albumin concentrations has been proposed (Pospisil J et al, 1994). In patients with end-stage renal disease (creatinine clearance less tahn 10 mL/min), the free fraction of phenytoin increases from 0.1 to approximately 0.2-0.35. The following equation is useful in predicting normalized phenytoin concentrations for this kind of patients: C obs C calcu 0.1. ALB 0.1 (Vd * Km * ln( Cp0 / Cpt ) Vd * (Cp0 Cpt ) Vmax The decline of phenytoin concentrations after discontinuation of therapy can be described as follows: t Where Cp0 is the initial plasma concentration and Cpt is the plasma concentration at the end of the time interval t. Factors affecting phenytoin plasma concentration are: Liver disease: acute hepatitis impairs the liver´s ability to metabolise phenytoin Hypoalbuminaemia: Chronic liver disease, nephrotic syndrome, pregnacy and some chronic illness can result in hypoalbuminemia which increases the free phenytoin fraction and resutls in an increased rate of its metabolism. Drug interactions: Inhibition of metabolism: drugs inhibiting hepatic mono-oxygenase activity may cause a reduction in the rate of phenytoin metabolism, which increase plasma drug concentration and increase risk of toxicity: Cimetidine Amidarone Chlorpromazine Imipramine Omeprazol Sulphonamides H2-Antoagonis Disulfiram Allopurinol Azapropazone Isoniazida Metronidazol Oral anticoagulants Thioridazine Antituberculosis drugs Stimulation of metabolism: drugs stimulating hepatic mono-oxygenase activity which results in a decrease in plasma phnytoin concentration and the consequent risk of breakthrought seizures, are the following: Carbamazepine Felbamate Tiagabine Diazepam Phenobarbital Lamotrigine Dexamethasone Chlordiazipoxide Rifampicine Topiramate Nitrofurantoin Displacement from protein binding: drugs displacing phenytoin from its binding sites on plasma albumin, increasing the fraction of drug unbound which alters the interpretation of the total plasma phenytoin concentration are: Aspirin Sodium Valproate ADVERSE EFFECTS They are related to plasma concentrations: Plasma levels (mg/L) Symptom 20-30 20-30 30-40 30-40 > 40 Mental changes Nystagmus Ataxia Long term adverse effects of Phenytoin Gingival hyperplasia Coarsening of facial features Acne Hirsutism Folate deficiency Vitamin D deficiency THERAPEUTIC RANGE Therapeutic range accepted, in the absence of complicating factors, is between 10-20 mg/L (total plasma level) but it should be only used as a guide to the treatment of epilepsy. This range changes for particular seizure syndromes and for patients with different degrees of seizure activity within each seizure syndrome type. So, there are evidence that bilateral tonic-clonic seizures are controlled by phenytoin at lower plasma concentration than those required to control partial seizures. Some patients can be completely controlled at plasma concentrations below 10 mg/L but other patients with severe epilepsy may require concentrations in excess to upper limit (20 mg/L) and patient tolerate the drug well. The therapeutic range of total plasma concentrations falls in renal failure and when a hypoalbuminaemia exits. Patients Adults and children Newborns and children under 3 years Endstage renal disease or hypoalbuminaemia Endstage renal disease and hypoalbuminaemia Liver disease Acute hepatitis Therapeutic range 10-20 mg/L 6-14 mg/L 5-10 mg/L 3-7 mg/L 7-14 mg/L 8-16 mg/L Since about 10 % of phenytoin is unbound in the plasma, a therapeutic range for free phenytoin of 1-2 mg/L has been recommended. Knowledge of the plasma concentration that produces a good response in the particular patient (the so-called "individual therapeutic concentration" will provide an important reference in making further dosage adjustment. INDICATIONS FOR MONITORING At the initiation of the therapy, in order to achieve a plasma concentration within the therapeutic range. The concentration at which seizure control has been attained without adverse effects serves as the reference value. When seizures reccur after a period of control in order to know if plasma concentration is lower than that control was obtained. When there is intercurrect illness (AIDS patients, hypoalbuminemia, renal impairment...) or a change in physiological state exists (age, pregnancy...) When drug toxicity is suspected When phenytoin therapy is about to be withdrawn Intervals during the course of therapy SAMPLING TIMES The timing of plasma sampling for phenytoin is not critical, because the fluctuation in its plasma concentration is relatively small, even when dosage is once daily. That is why individual sample is usually drawn at steady-state and at the end of the dosage interval. This concentration is minimally influenced by variations in drug absorption rate and it can be assumed that aproximates the average steady-state concentrations. In those patients requiring rapid achievement and maintenance of therapeutic phenytoin concentrations , it is usal to monitor phenytoin plasma level within two to three days of therapy initiation (non steady-state situation). KINETIC MODEL One-compartment model with zero order absorption and Michaelis-Menten elimination. DOSAGE RECOMMENDATIONS Population Adults Children < 3 months Children 6 months-6 years Pregnancy Maintenance dose 5-7 mg/Kg/day 3-5 mg/Kg/day 5-15 mg/Kg/day 5-15 mg/Kg/day Dosage interval 12 (8, 12 and 24) hours 8 hours 12 (8 and 12) hours 12 (8 and 12) hours Small changes in phenytoin dosage may cause large changes in plasma phenytoin concentrations, so they should be done with precaution. There are a number of graphic approaches so-called "nomograms" that can be used to estimate individual dosage requirements. They require information from one, two or more steady-sate plasma concentrations in the patient and may be a practical aid in adult and pediatric practice Clinical utility shown of Bayesian forecasting methods: acceptable predictive performance in non-steady-state and steady-state situations. OBSERVATIONS Monitoring of free phenytoin concentrations may be beneficial in patients with AIDS, hypoalbuminemia and renal impairment. Besides, when the patient receive another drug such as Valproic acid which can compete with phenytoin for protein binding. Time to reach steady-state increases with dose. The time required to achieve 90% of t90% Km.Vd * 2.3 *Vmax 0.9( Dose / day) (Vmax ( Dose / day)) 2 steady state, can be calculated as follows: A nomogram has been developed to help the physician to decide whether a measured plasma concentration can be regarded in steady-state situation. Long term treatment with phenytoin may result in a folate dependent megaloblastic anemia, which is treated with folic acid (folic acid increasess phenytoin clearance) so plasma phenytoin conentration will be reduced and this may result in breakthrough seizures. There is a complicated interaction between phenytoin and sodium valproate which displaces phenytoin from protein binding sites and thus changes the therapeutic range, but also inhibits phenytoin metabolism, making difficult to interpret plasma phenytoin concentrations in patients taking valproate. POPULATION MODELS REFERENCES Alonso AC, García MJ, Domínguez-Gil A. Contribution of serum level monitoring in the individualization of carbamazepine dosage regimens. Int J Clin Pharmacol Ther Toxicol 1988; 26: 409-12. Alonso AC, García MJ, Domínguez-Gil A. Intra and interindividual relationship between serum levels and dose in epileptic patients treated with carbamazepine monotherapy. Ther Drug Monit 1988; 10: 501-3. Alonso González AC, Ortega Valin L, Santos Buelga D, García Sánchez MJ, Santos Borbujo J, Monzon Corral L, Domínguez-Gil Hurlé A. Dosage programming of phenobarbital in neonatal seizures. J Clin Pharm Therap 1993; 18: 267-70. Alonso AC, Santos D, García MJ, Domínguez-Gil A. Utilidad de los métodos bayesianos en la terapéutica antiepiléptica. Rev Esp Epilepsía 1993; 4: 10-3. Armijo JA, Cavada E. Graphic estimation of phenytoin dose in adults and children. Ther Drug Monitor 1991; 13:507-10. Battino D, Estienne M, Avanzini G. Clinical pharmacokinetics of antiepileptic drugs in paediatric patients. Part I: Phenobarbital, primidone, valproic acid, ethosuximide and mesuximide. Clin Pharmacokinet 1995; 29: 257-86. Battino D, Estienne M, Avanzini G. Clinical pharmacokinetics of antiepileptic drugs in paediatric patients. Part II: Phenytoin, carbamazepine, sulthiame, lamotrigine, vigabatrin, oxcarbazepine and felbamate. Clin Pharmacokinet 1995; 29: 341-69. Bertilsson L, Tomson T. Clinical pharmacokinetics and pharmacoligical effects of carbamazepine and carbamazepine 10-11 epoxide. An update. Clin Pharmacokinet 1986; 11: 177-98. Bialer M. Pharmacokinetic evaluation of sustained release formulations of antiepileptic drugs. Clinical Implications. Clin Pharmacokinet 1992; 22: 11-21. Bialer M. Comparative pharmacokinetics of the newer antiepileptic drugs. Clin Pharmacokinet 1993; 24: 441-52. Brodie MJ, Drug interactions in epilepsy. Epilepsia 1992; 33(Suppl 1): S13-S22. Brown C. Handbook of Drug Therapy Monitoring. Willians & Wilkins. Baltimore. 1990 Caraco Y, Zylber-Katz E, Levy M. Carbamazepine pharmacokinetics in obese and lean subjects. Ann Pharmacother 1995; 29: 843-7 Cloyd J et al. Valproic acid pharmacokinetics in children. IV: Effects of age and antiepileptic drugs on protein binding an intrinsic clearance. Clin Pharmacol Ther 1993. 1:22-9. Crawford P, Chadwick DJ, Martin C. et al. The interaction of phenytoin and carbamazepine with combined oral contraceptive steroids. Br J Clin Pharmac 1990; 30: 892-6. Choonara IA, Rane A. Therapeutic drug monitoring of anticonvulsants: state of the art. Clin Pharmacokinet 1990; 18: 321-8. Duncan JS, Patsoles PN, Shorvon S. Effects of discontinuation of phenytoin, carbamazepine and valproate on concomitant antiepileptic medication. Epilepsia 1991; 32: 101-15. Encinas MP, Santos Buelga D, Alonso González AC, García Sánchez MJ, DomínguezGil Hurlé, A. Influence of lenght of treatment on the interaction between phenobarbital and phenytoin. J Clin Pharm Therap 1992; 17: 49-50. Eadie MJ. Formation of active metabolites of anticonvulsant drugs. A reiew of their pharmacokinetic and therapeutic significance. Clin Pharmacokinet 1991; 21: 27-41. Eadie MJ. Plasma antiepileptic drug monitoring in a neurogical practice: a 25-years experience. Ther Drug Monit 1994; 16: 458-68. Evans WE et al. Applied Pharmacokinetics. Principles of therapeutics drug monitoring. 3ª Ed. Applied Therapeutics, Inc. Vancouver.1992. Fernández de Gatta MR, Alonso AC, García MJ, Domínguez-Gil A. Effect of sodium valproate on phenobarbital serum levels in children and adults. Ther Drug Monit 1986; 8: 416-20. García MJ, Alonso AC, Domínguez-Gil A. Monitorización de antiepilépticos: (I) Iniciación de una terapéutica antiepiléptica. Revista AEFH 1986; 10: 41-8. García MJ, Alonso AC, Domínguez-Gil A. Monitorización de antiepilépticos: (II) Fármacos que se ajustan a una cinética lineal. Revista AEFH 1986; 10: 73-80. García MJ, Alonso AC, Domínguez-Gil A. Monitorización de antiepilépticos: (III) Aplicación de la cinética michaeliana en los tratamientos con difenilhidantoína. Revista AEFH 1986; 10: 217-23. García Sánchez MJ, Alonso González AC, Maza A, Santos Buelga D, Dóminguez-Gil Hurlé, A. Comparison of methods of carbamazepine dosage individualization in epileptic patients. J Clin Pharm Therap 1988; 13: 375-80. Gómez MJ, Santos Buelga D, Alonso AC, García MJ, Domínguez-Gil A. Effect of dose on the kinetic behaviour of valproic acid: modifications in plasma protein binding. Eur J Drug Met Pharmacokient. 1991; 3: 338-42. Gómez MJ, García MJ, Alonso AC, Santos D, Domínguez-Gil A. Plasma protein binding kinetics of valproic acid over a broad dosage range: Therapeutic implications. J Clin Pharm Ther 1993; 18: 191-7. Gram L, Flachs H, Wurtz-Jorgensen A, Parnes J, Anderson B. Sodium valproate, relationship between serum levels and therapeutic effects: A controlled study. En: Antiepileptic Therapy: Advances in drug monitoring, Johannesssen SI, Morselli PL, Pippenger CE, Richens A, Schmidt D, Meinardi H (Eds.). Raven Press, New York, 1980; 247-52. Reynolds C. The place of saliva in antiepileptic drug monitoring. Ther Drug Monit 1984; 6: 35-41. Lai ML, Lin TS Huang. Effects of single and multiple dose carbamazepine on the pharmacokinetics of diphenylhidantoin. Eur J Clin Pharmacol 1992; 43: 201-3. Larkin JG, Herrick AL et al. Antiepilectic drug monitoring at the epilepsy clinic: A prospective evaluation. Epilepsia. 1991; 32: 89-95. Ledesma A, Acosta A, Izquierdo JA, García MJ, Domínguez-Gil A. Electroclinical correlations with plasma and saliva levels of anticonvulsivantes in epileptic patients. Pronesis 1984; 5: 55-8. Levi RH. et al. Antiepileptic drugs. 3rd ed. New York. Raven Press. 1989. Levine M, Orr J, Chang T. Evaluation of nomogram for estimating the rate of pheynytoin accumulation. Ther Drug Monit 1987; 9: 166-70. Levine M, Chang T. Therapeutic drug monitoring of phenytoin. Rationale and current status. Clin Pharmacokinet 1990; 19: 341-58. Liponi DF, Winter ME, Tozer TN. Renal function and therapeutic concentrations of phenytoin. Neurology 1984; 34: 395-7. McKee PJW, Larkin JG, Brodie AF, Percy-Robb IW, Brodie MJ. Five years of anticonvulsants monitoring on site at the epilepsy clinic. Ther Drug Monit. 1993; 15: 8390. Nation RL, Evans AM, Milne RW. Pharmacokinetics drug interactions with phenytoin (Part I). Clin Pharmacokinet 1990; 18: 37-70. Panesar SK, Orr JM, Farrel K. et al. The effect of carbamazepine on valproic acid disposition in adults volunteers. Br J Clin Pharmacol 1989; 27: 323-8. Pitlick WH, Levy RH, Troupin AS, Grenn JR. Pharmacokinetic model to describe selfinduced decreases in steady-state concentrations of carbamazepine. J Pharmac Sci 1976; 65: 462-3. Pospisil J, Pelclova D. A graphical nomogram method for predicting toxic concentrations of unbound phenytoin. I J Clin Pharmacol Ther 1994; 32: 122-5. Pryka RD, Rodvold KA, Erdman SM. An updated comparison of drug dosing methods. Part I: Phenytoin. Clin Pharmacokinet 1991; 20(3): 209-17. Pugh CB, Garnett W. Current issues in the treatment of epilepsy. Clinical Pharmacy 1991; 10: 335-58. Richens A, Dunlop A. Serum phenytoin levels in the management of epilepsy. Lancet 1975; 2: 247-8. Riva R, Albani F, Contin M, Baruzzi A. Pharmacokinetic interactions between antiepileptic drugs. Clinical considerations. Clin Pharmacokinet 1996; 31: 470-93. Suzuki Y, et al. Phenytoin age-dose concentration relationship in children. Ther Drug Monitor 1994; 16: 145-50. Thomson AH, Brodie M. Pharmacokinetic optimization of anticonvulsant therapy. Clin Pharmacokinet 1992; 23: 216-30. Vozeh S, Follath F. Nomographic estimation of time to reach steady-state serum concentration during phenytoin therapy. Eur J Clin Pharmacol 1980; 17: 33-5. Weidle PJ, Skiest DJ, Forrest A. Multiple-dose activated charcoal as adjunt therapy after chronic phenytoin intoxication. Clin Pharm 1991; 10: 711-4. Wilensky AJ, Frield PN, Levy RH, Confort CP, Kaluzny SP. Kinetics of phenobarbital in normal subjects and epileptic patients. Eur J Clin Pharmacol 1982; 23: 87-92. Yukawa E. Optimisation of antiepileptic drug therapy. The importance of serum drug concentration monitoring. Clin Pharmacokinet 1996; 31: 120-30. Zaccara G, Messori A, Moroni F. Clinical pharmacokinetics of valproic acid-1988. Clin Pharmacokinet 1988; 15: 367-89.