Determine the relative molecular mass of a volatile liquid
advertisement

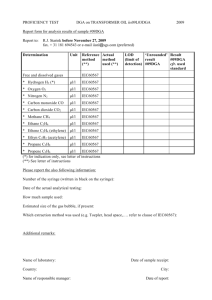
Determine the relative molecular mass of a volatile liquid. initial mass of hypodermic syringe containing ethyl ethanoate=19.198g final mass of hypodermic syringe containing ethyl ethanoate=18.975g Atmospheric pressure=101.3kPa initial volume of graduated gas syringe=l0.0cm3 final volume of graduated gas syringe =85.9cm3 Temperature measured=100oC a) Calculate the relative molecular mass of ethyl ethanoate. P (V2 – V1) = n R T = (m/M) R T (101.3X103)[(85.9-10.0)/106] = [(19.198-18.975)/M] (8.314 )(100+273) M = 89.9 b) A student took an initial reading of the graduated gas syringe as 0.0cm3. Comment whether he is right or wrong. Explain. Wrong--Empty space is required for injecting ethyl ethanoate liquid into the graduated gas syringe. c) List some possible sources of errors in this experiment. --The vapour produced by the volatile liquid may not behave ideally, i.e. it may not obey the ideal gas law at the experimental conditions. --There is a great error in the measurement of the mass of volatile liquid injected into the syringe(because the mass is very small). --There is a possible error in recording the temperature (because the temperature in the steam jacket may differ from that of the vapour inside the gas syringe). --Friction caused by the piston of the large graduated syringe. d) Suggest THREE precautions in this experiment. -Rinse the hypodermic syringe with the volatile liquid to ensure smooth filling. -Expel all air bubbles from the hypodermic syringe to ensure that it is completely filled with the volatile liquid. -Wait for a steady temperature before injecting propanone into the graduated gas syringe. -Wait for a steady volume reading, thereby ensuring the complete vaporization of propanone e)A student use the same apparatus above to determine the relative molecular mass of butan-1-ol(boiling point is 118oC). Explain whether he could get an accurate result or not. He could not. The butan-1-ol(boiling point is 118oC) cannot be evaporated completely at the steam temperature (100oC). Most of the butan-1-ol liquid remained as liquid(not evaporated).