Lethal Protein
advertisement

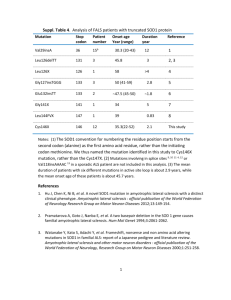
Lethal Mutation Project Overview Discipline: Science Course: Genetics RWLO Description: Students will use the NCBI website (www.ncbi.nlm.nih.gov) to research a genetic disorder, ALS, and the mutant protein, SOD1, which causes the disorder. Students will then download the protein structures of both the normal and two mutant SOD1 proteins and view them in 3D using downloadable viewing software called Cn3D. Students will describe the effect of the mutations in terms of the effect on protein structure. Students will be able to correlate the molecular mutation to the physiological symptoms of the disease. The National Center for Biotechnology Information (NCBI) is a division of the National Library of Medicine (NLM) at the National Institutes of Health (NIH). The NCBI maintains many enormous databases, including GenBank, which stores all known public DNA sequences, submitted from individual scientists and large genome centers around the world; OMIM, a Web-based catalog that contains thousands of entries for genes and genetic disorders, which is frequently updated by NCBI staff based on published work in the scientific community; the Molecular Modeling Database (MMDB) of 3D protein structures; and many, many others. No other source of up-to-date molecular biology information is even remotely of the size and scope of this collection of resources. Audience: Undergraduate students in genetics (or other biology) courses studying the effects of gene mutation on protein structure. Context information: Students should be knowledgeable on the four levels of protein structure (primary, secondary, tertiary, and quaternary) including the types of bonds that stabilize or define each level. 1 Student Learning Objectives For this RWLO, the student will be able to: Use the NCBI website to research ALS and the protein SOD1. Use a downloaded viewer program to view the 3D structure of the normal protein and two mutant proteins. Make comparisons of the normal structure with the mutant structure in terms of the four levels of protein structure. Explain how a specific gene mutation creates a protein with a mutant 3D structure. Relate the mutant protein structure to the physiological symptoms of the disease. 2 Procedure Time: Approximately 45 minutes Materials: Computer with internet connection Prerequisites: Review of the central dogma of biology, gene mutation, and protein structure. Implementation: This RWLO can be used as a homework assignment to reinforce and/or demonstrate how a single nucleotide mutation in a gene affects the structure of the resulting protein, and in turn how this altered structure creates a physiological malfunction (genetic disease). This RWLO could also be used in the classroom as a demonstration by the instructor. Steps: Download the 3D protein viewer: 1. Click here to begin. 2. Click on “Download Cn3D 4.1 for PC, Mac, and Unix” and follow the instructions. (This will download the viewing software you will need to view a protein in 3D on your screen.) Now you are ready to begin the activity: 3. Go to OMIM. Search OMIM for “SOD1.” Your results: The first entry is *147450, Superoxide dismutase 1, SOD1 The second entry is #105400, Amyotrophic Lateral Sclerosis 1; ALS1 Start with the second entry first: 4. Click on the #105400 (second entry) to gather background information on ALS, the disease caused by mutant SOD. 5. Have students focus on Paragraphs 1,2, 10, and 14 to get an overview of key points. 6. Students will answer the following questions: A. 15 to 20% of cases of familial amyotrophic lateral sclerosis type 1 (ALS1) are associated with a mutation in what gene? SOD1 3 B. Familial forms of ALS follow what mode of transmission? Autosomal dominant C. What is another name for ALS? Lou Gehrig’s disease D. What is the nature of the symptoms and the disease progression? Loss of motor function with predominantly lower motor neuron manifestations. Wasting of muscles of 1 hand and spreading of the disorder in a contiguous manner. Lower motor neuron involvement is usually conspicuous, whereas involvement of upper motor neurons is less marked. E. Does the disease show incomplete penetrance and/or variable expressivity? Highly variable in terms of age of onset and duration of the disease. By age 85 years, about 80% of carriers have manifested the disorder. F. Why is euthanasia an issue involved with ALS? The progressive paralysis leads to increase of loss of function, culminating in complete dependence on the help of others for all activities of daily living and, if life is sustained by assisted ventilation, loss of the ability to communicate or swallow. G. What is the title of the bestselling book by Mitch Albom, describing the last year in the life of an ALS patient? Tuesdays with Morrie 7. Use the back arrow button to return to the OMIM results list. 8. Review entry *147450 (regarding SOD1) paragraphs 3, 13, and 16 and answer these questions: A. What reaction is catalyzed by superoxide dismutase? Disproportionation or dismutation of superoxide radicals to molecular oxygen and hydrogen peroxide. B. What scientist identified the first disease-producing mutations in SOD1? Rosen in 1993 C. What type of mutations were found? Missense D. Describe the two proposed mechanisms by which mutant SOD1 would cause cellular damage in ALS patients. i. Hypoactivity of SOD1 could lead to a build-up of the toxic superoxide radical. ii. Increased activity of SOD1 could lead to excessive levels of H2O2 and a highly toxic hydroxyl radical. E. What specific mutation was found to be most frequent? Ala4val or A4V 4 F. Examination of the crystal structure of human SOD established what? “Examination of the crystal structure of human SOD established that all 12 observed sites of mutation causing ALS alter conserved interactions critical to the beta-barrel fold and dimer contact, rather than catalysis.” 9. Click on “Links” for SOD1 (to the far right), and scroll down to “Structure” and click. This takes us to the structure database. We will be using the following entries: 1UXM A4V mutation of human SOD1 1UXL I113T mutation of human SOD1 1SPD Human superoxide dismutase 1 (SOD1) 10. First click on 1SPD, the normal structure of SOD1, and on the next screen click on “View 3D Structure” (the gray button). The viewing program should open up a new window with the structure of SOD1. Note that you can click and drag on the image to rotate it in 3D. Answer the following questions: A. What secondary structures are visible? How many can you find of each type? SOD1 is a dimer, so each subunit contains 10 beta sheets, although the students may count 20 if they don’t yet notice it is a dimer. There are no alpha helices. B. What metal cofactors are present? Copper and zinc. C. Locate the amino and carboxyl termini. What does this tell you about quaternary structure? There are four “ends” which means there are 2 subunits here, so it is a dimer. 11. Now click on 1UXM, view the structure and answer these questions: A. Which level of protein structure is visibly altered by the A4V mutation, compared to the normal SOD1 structure? Quaternary structure – the mutant SOD1 is a polymer, not a dimer. 12. Now click on 1UXL, study the structure and answer these questions: A. 1UXM is described as an “A4V mutation of human SOD1.” This means that the fourth amino acid in the protein has been changed from adenine to valine, due to a missense mutation in the gene. 1UXL is described as an “I113T mutation of human SOD1.” What does this mean? The 113th 5 amino acid has been changed by missense mutation from isoleucine to threonine. B. Which level of protein structure is visibly altered by the I113T mutation, compared to the normal SOD1 structure? Again, quaternary structure – the mutant SOD1 is also a polymer, not a dimer. 13. Close the structure viewing windows and use the back arrow to return to the structures list. On the right side of the 1UXM entry, click on “Links” and drag down to “Full text in PMC.” This will take you to a list of 2 journal articles which study the A4V and I113T mutations. Click on the “Abstract” of each article, read through them quickly, and answer these questions: A. What can you find out about the location of the A4V and I113T mutations, in terms of both tertiary and quaternary structure? They exist at the “dimer interface” – the part of the subunit that interacts or bonds with the other subunit. B. What effect do these mutations have on the dimer formation? They destabilize the dimer and result in aggregation of the subunits. Instructor: Be sure to emphasize that the aggregates cannot function properly, thus the superoxide anions build up and become toxic to the cells. Because higher levels of SOD1 are produced in motor neurons, these cells show the effects first. The death of motor neurons results in the symptoms of ALS described above. 14. Students complete the activity and all questions prior to the next class period. The instructor might spend 10-15 minutes at the beginning of the next class answering questions and giving any clarifications needed. Or, if preferred, the students can form groups of 3-4 to discuss the activity. 15. To complete the project, students should use their answers to all the previous questions to synthesize a 1-page written summary describing how mutation of the SOD1 gene relates to the disease ALS. The summary should include a description of ALS on both a physiological and cellular level and the mode of transmission in familial cases; the normal structure and function of SOD1 protein in a motor neuron; the types of SOD1 mutations associated with ALS; and an explanation of how these mutations affect the structure of SOD1 and thus cause neuronal death. The summary should be 3/4 page typed, 250-300 words. 6 Content Material Student Directions: Time Required: Approximately 45 minutes Materials: Computer with internet connection Prerequisites: Review of the central dogma of biology, gene mutation, and protein structure. Steps: Download the 3D protein viewer: 1. Click here to begin. 2. Click on “Download Cn3D 4.1 for PC, Mac, and Unix” and follow the instructions. (This will download the viewing software you will need to view a protein in 3D on your screen.) Now you are ready to begin the activity: 3. Go to OMIM. Search OMIM for “SOD1.” Your results: The first entry is *147450, Superoxide dismutase 1, SOD1 The second entry is #105400, Amyotrophic Lateral Sclerosis 1; ALS1 Start with the second entry first: 4. Click on the #105400 (second entry) to gather background information on ALS, the disease caused by mutant SOD. 5. Focus on Paragraphs 1,2, 10, and 14 to get an overview of key points. 7 6. Answer the following questions: a. 15 to 20% of cases of familial amyotrophic lateral sclerosis type 1 (ALS1) are associated with a mutation in what gene? ________________________________________________________________ b. Familial forms of ALS follow what mode of transmission? ________________________________________________________________ c. What is another name for ALS? ________________________________________________________________ d. What is the nature of the symptoms and the disease progression? ________________________________________________________________ e. Does the disease show incomplete penetrance and/or variable expressivity? ________________________________________________________________ f. Why is euthanasia an issue involved with ALS? ________________________________________________________________ ________________________________________________________________ g. What is the title of the bestselling book by Mitch Albom, describing the last year in the life of an ALS patient? ________________________________________________________________ 7. Use the back arrow button to return to the OMIM results list. 8 8. Review entry *147450 (regarding SOD1) paragraphs 3, 13, and 16 and answer these questions: a. What reaction is catalyzed by superoxide dismutase? ________________________________________________________________ b. What scientist identified the first disease-producing mutations in SOD1? ________________________________________________________________ c. What type of mutations were found? ________________________________________________________________ d. Describe the two proposed mechanisms by which mutant SOD1 would cause cellular damage in ALS patients. ________________________________________________________________ e. What specific mutation was found to be most frequent? ________________________________________________________________ f. Examination of the crystal structure of human SOD established what? ________________________________________________________________ 9. Click on “Links” for SOD1 (to the far right), and scroll down to “Structure” and click. 10. This takes us to the structure database. We will be using the following entries: 1UXM A4V mutation of human SOD1 1UXL I113T mutation of human SOD1 1SPD Human superoxide dismutase 1 (SOD1) 11. First click on 1SPD, the normal structure of SOD1, and on the next screen click on “View 3D Structure” (the gray button). The viewing program should open up a new window with the structure of SOD1. Note that you can click and drag on the image to rotate it in 3D. 9 12. Answer the following questions: a. What secondary structures are visible? How many can you find of each type? ________________________________________________________________ b. What metal cofactors are present? ________________________________________________________________ c. Locate the amino and carboxyl termini. What does this tell you about quaternary structure? ________________________________________________________________ 13. Now click on 1UXM, view the structure and answer these questions: a. Which level of protein structure is visibly altered by the A4V mutation, compared to the normal SOD1 structure? ________________________________________________________________ 14. Now click on 1UXL, study the structure and answer these questions: a. 1UXM is described as an “A4V mutation of human SOD1.” This means that the fourth amino acid in the protein has been changed from adenine to valine, due to a missense mutation in the gene. 1UXL is described as an “I113T mutation of human SOD1.” What does this mean? ________________________________________________________________ b. Which level of protein structure is visibly altered by the I113T mutation, compared to the normal SOD1 structure? ________________________________________________________________ ________________________________________________________________ 10 15. Close the structure viewing windows and use the back arrow to return to the structures list. On the right side of the 1UXM entry, click on “Links” and drag down to “Full text in PMC.” This will take you to a list of 2 journal articles which study the A4V and I113T mutations. Click on the “Abstract” of each article, read through them quickly, and answer these questions: a. What can you find out about the location of the A4V and I113T mutations, in terms of both tertiary and quaternary structure? ________________________________________________________________ ________________________________________________________________ b. What effect do these mutations have on the dimer formation? ________________________________________________________________ ________________________________________________________________ 16. Homework (check with your instructor for due date): Use your answers to all the previous questions in this activity to synthesize a 1-page written summary describing how mutation of the SOD1 gene relates to the disease ALS. The summary should include a description of ALS on both a physiological and cellular level and the mode of transmission in familial cases; the normal structure and function of SOD1 protein in a motor neuron; the types of SOD1 mutations associated with ALS; and an explanation of how these mutations affect the structure of SOD1 and thus cause neuronal death. The summary should be 3/4 page typed, 250-300 words. Referenced URLs: www.ncbi.nlm.nih.gov 11 Assessment Class Session 1: Instructor introduces the activity and distributes the content materials. Instructor emphasizes that the last item (#16 “Homework”) will not be due at the next class period. Students will complete the questions found in the activity as they perform the tasks. Class Session 2: Students will bring the content materials with the completed questions to class for clarifications. 50% of the total points are awarded for the student bringing the completed questions and participating in the discussion group. Instructor assigns the #16 homework essay. Class Session 3: The remaining 50% of points for this activity will be awarded for the essay. Student essays will be graded based on content requirements as well as clarity, use of scientific terms and language, and overall quality. A sample rubric is given on the following page. 12 Assessment Grading Rubric for Student Essays Assignment Criteria 5 Essay addresses all components of the assignment, with evidence of independent thought. 4 Essay addresses all components of the assignment. 3 Subject is clear—although essay misses some required components. 2 Subject is generally clear but not in keeping with assigned topic. 1 Essay is not in keeping with assigned topic. Organization and Flow of Thought 5 Essay shows a logical relationship of related ideas in coherent, sequential paragraphs. 4 Essay shows good development of ideas in coherent, sequential paragraphs. 3 There is evidence of a sequence of ideas, but paragraph structure is faulty. 2 Essay relies on vague arguments and unsupported information. 1 Essay is incoherent, with little development of relationships among ideas. Language Usage 5 Student has edited the essay, ensuring that sentences are clear and logical, with appropriate language and no grammar or punctuation errors. 4 Student has edited the essay, but a few minor errors remain. 3 The style is generally weak and a moderate amount of errors remain. 2 Serious style and language errors remain. 1 The essay is stylistically inappropriate. TOTAL POINTS Assignment Criteria Organization Language Usage Total Points /15 pts 13 Links to Course Competencies This RWLO could be applied in the following courses: Introductory Biology, Genetics, and others. Specifically, this RWLO meets the following course competencies: Compliance with Core Curriculum Guidelines Intellectual Competencies: 1. Reading: the ability to analyze and interpret a variety of printed materials-books, documents, and articles- above the 12th grade level. 2. Writing: the ability to produce clear, correct and coherent prose adapted to purpose, occasion and audience-above the 12th grade level. 3. Speaking: ability to communicate orally in clear, coherent, and persuasive language appropriate to purpose, occasion, and audience –above the 12th grade level. 4. Listening: analyze and interpret various forms of spoken communication, possess sufficient literacy skills of writing, reading- above the 12th grade level. 5. Critical Thinking: think and analyze at a critical level. 6. Computer Literacy: understand our technological society, use computer-based technology in communication, solving problems, and acquiring information. Exemplary Educational Objectives: 1. To understand and apply method and appropriate technology to the study of natural sciences. 2. To recognize scientific and quantitative methods and the differences between these approaches and the other methods of inquiry, and to communicate findings, analyses, and interpretation both orally and in writing. 3. To identify and recognize the differences among competing scientific theories. 4. To demonstrate knowledge of the major issues and problems facing modern science, including issues that touch upon ethics, values, and public policies. 5. To demonstrate knowledge of the interdependence of science and technology, and their influence on, and contributing to, modern culture. 14 Supplementary Resources ALS Association: http://www.alsa.org/ An online database of mutations in ALS-related genes, including SOD1: http://alsod1.iop.kcl.ac.uk/ 15 Recommendations Recommendations for Integration: This activity is best integrated into the course with some components completed in the classroom and some as homework assignments. Alternatively, the instructor can use the entire RWLO as a demonstration for use in the classroom. This RWLO should be integrated into a series of lessons explaining the mechanisms of gene expression and gene mutation. The RWLO provides students with a “case study” of the SOD1 gene and how it, when mutated, causes a fatal disease. This reinforces the lessons with a real-life example. Back-up: The Cn3D viewing software and the 3 protein structure files can be saved to hard drive or disk ahead of time, in the case that internet access becomes unavailable. The procedures for the instructor and content material for students could be made available in hard copy in the case that RWLO files become corrupted or inaccessible. Advice to the Instructor: This RWLO could be adapted to other genes and genetic disorders. In order to do so, you must locate the structure files for both the normal protein and at least one mutant protein. However, a keyword search of the “Structure” database at the NCBI website tends to return far too many structure files to be manageable. I recommend that you first search OMIM for the gene name, then click and drag down the list under “Links” (to the right of the gene entry) until you find “Structure.” This path will return a much smaller number of relevant structure files. 16