Isolation of Taq polymerase from pTaq plasmid
advertisement

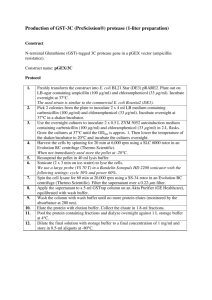
Isolation of Taq polymerase from pTaq plasmid Procedure taken from Biotechniques 9:780-784 (Nov 1995) 1. Inoculate 10 ml of LB with 100 ug Ampicillin/ml with 10 ul of overnight culture 2. Grow at RT overnight with shaking 3. Between 3-6 pm check OD600 of culture. Dilute to ~OD600 0.3 and a final volume of 5 mls LB. Add 1.0 M IPTG to a final concentration of 0.5 mM. 4. Grow overnight at 37oC with shaking (warm room 4th floor). 5. Spin down 1.5 ml of cells in two tubes (3 mls total) at full speed in microfuge for 5 minutes 6. Add 4 mg/ml lysozyme (final) to Buffer A 7. Resuspend each tube into 45 l Buffer A with lysozyme 8. Incubate at RT for 15 minutes 9. Add 45 l Buffer B and incubate 60 minutes at 75oC 10. Centrifuge debris at full speed for 10 min 11. Save supernatant. Add 2 volumes of the storage buffer. 12. Freeze aliquots at –20oC. Make 2 mls of each buffer and filter sterilize Buffer A Buffer B Stock solution 1 M Tris-HCl (pH 7.9) 1 M Glucose 0.5 M EDTA Sigma H20 Final concentration [50 mM ] [50 mM ] [1 mM ] 1 M Tris-HCl (pH 7.9) 2 M KCl 0.5 M EDTA Tween 20 Nonidet P-40 Sigma H20 [10 mM ] [50 mM ] [1 mM ] [0.5% ] [0.5% ] Storage Buffer 1 M Tris-HCl (pH 7.9) 2 M KCl 0.5 M EDTA 100 mg/ml of BSA 0.5 M DTT Glycerol Sigma H20 [20 mM ] [50 mM ] [0.1 mM ] [1 mg/ml ] [1 mM ] [50% ] You need to know the answers to these questions: What are special about the cells? Why treat with IPTG? What happens at the Buffer A step? What happens in the Buffer B step? Why are the following in the storage buffer? KCl BSA Glycerol -