20100520-jcp-SI
advertisement

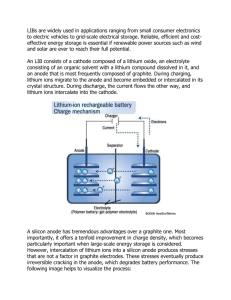
Supporting Information Lithium Transport at Silicon Thin Film: Barrier for High-rate Capability Anode Bo Peng, Fangyi Cheng, Zhanliang Tao, and Jun Chen* Institute of New Energy Material Chemistry and Key Laboratory of Advanced Micro/Nanomaterials and Batteries/Cells (Ministry of Education), Chemistry College, Nankai University, Tianjin 300071, P.R. China * To whom correspondence should be addressed. E-mail: chenabc@nankai.edu.cn This material contains Figure S1 , Figure S2, and Table S1. Figure S1. (a) Cutoff energy test of Si bulk; (b) Lattice constant predicted by GGA vs. experimental value (error < 1%); (c–e) k-points test of Si unit cell (c), 3×3×3 Si supercell (d), and c(4×4) Si (100) (e). Figure S2. Full Li transport path profile in B-doped case. Table S1. Possible species that may exist at the surface of silicon anode and their properties during the electrochemical process. Species -Si-O Property SiO2, -Si-O-Si-, -Si-OH React with the organic solvent, lithium salt, organic additive or impurities, which is one of the major reasons of initial irreversible capacity loss.1 -Si-OCH2CH2OCO2Li Less dense , decomposable, through which the electrolyte can penetrate and break Si-Si network (cracking).2 -Si-F With large bond energy (565 kJ mol-1) but high reactivity due to a kinetics-driven surface chemistry,3 thus the existence of Si-F at the surface is doubted. -Si-C Experimentally, to avoid these undesirable species, some additives (e.g. carbon) are introduced to result in stable solid-electrolyte-interface (SEI). For example, coating Si-anode with carbon layer helps to enhance the electronic conductivity and to keep the anode remaining integrated after cycling.4 However, for a carbon-coated Si-anode with the thickness of carbon layer less than the XPS penetration depth (~10 nm), no obvious signal of interfacial Si-C bond could either be observed from Si(2p3/2) or from C(1s) X-ray photoelectron spectroscopy (XPS).4,5 Thus, Si-C is Less possibly located on the surface of nanostructured silicon. According to Ref. 4 and 6, Si-C exists with the form of siloxane (C-Si-O), which may be soluble in the electrolyte and subsequently diffuse through the SEI layer to deposit on the top of carbon layer. -Si-H Reactive and can chemically reduce the electrolyte References [1] Graetz, J.; Ahn, C. C.; Yazami, R.; Fultz, B. “Highly reversible lithium storage in nanostructured silicon” Electrochem. Solid-State Lett. 2003, 6, A194-A197. [2] Choi, N.-S.; Yew, K. H.; Lee, K. Y.; Sung, M.; Kim. H.; Kim, S.-S. “Effect of fluoroethylene carbonate additive on interfacial properties of silicon thin-film electrode ”J. Power Sources 2006, 161, 1254-1259. [3] Ren, B.; Liu, F. M.; Xie, J.; Mao, B. W.; Zu, Y. B.; Tian, Z. Q. “In situ monitoring of Raman scattering and photoluminescence from silicon surfaces in HF aqueous solutions” Appl. Phys. Lett. 1998, 72, 933. [4] Yen, Y.-C.; Chao, S.-C. ; Wu, H.-C. ; Wu, N.-L. “Study on solid-electrolyte-interphase of Si and C-coated Si electrodes in lithium cells” J. Electrochem. Soc. 2009, 156, A95-A102. [5] Kim, H.; Han, B.; Choo, J.; Cho, J. Angew. Chem. Int. Ed. 2008, 47, 10151–10154. [6] Chan, C.-K.; Ruffo, R.; Hong, S.-S.; Cui, Y. “Surface chemistry and morphology of the solid electrolyte interphase on silicon nanowire lithium-ion battery anodes” J. Power Sources 2009, 189, 1132-1140.