Word file (415 KB )
advertisement

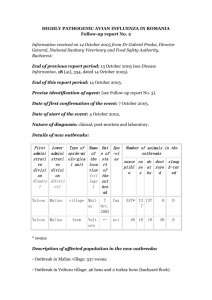
Methods Samples. Between January 19th and March 10th 2006, cloacal swabs were collected from 118 flocks and 100 commercial farms (cockerels, broilers, pullets, layers and breeders) in Oyo, Ogun and Lagos states (for Fig. 1a) in South-Western Nigeria within the framework of the Nigerian-Luxembourg Poultry Virus Surveillance Network involving scientists from both countries as well as veterinarians and farmers from South-Western Nigeria. Flock sizes ranged from 125 to 20000 birds, ages ranged from 3 weeks to 2 years. We thank Dr Oyewola, president of the Oyo state branch of the Nigerian Veterinary Medical Association, Dr Oriade, head of the poultry unit of Oyo state veterinary Hospital Mokola, Ibadan, Dr Hassan, president of the National Farmers Association, and the participating farmers in Nigeria for their help with sample collection. RNA isolation and RT-PCR. RNA was isolated using a MagnaPure LC system with the MagnaPure LC Total nucleic acid isolation kit (Roche Diagnostics, Almere, The Netherlands) and influenza A virus was detected using a real-time RT-PCR assay. To ensure efficient influenza A virus detection, the published probe sequence was changed to 5’6-FAM-TTTGTG-TTC-ACG-CTC-ACC-GTG-CC-TAMRA-3’, based on the avian influenza A virus sequences available from public databases. Amplification and detection was performed on an ABI7700 machine with the TaqMan EZ RT-PCR Core Reagents kit (Applied Biosystems, Nieuwerkerk a/d IJssel, the Netherlands) using 20 l of eluate in an end volume of 50 l. For influenza A virus positive samples, a second reaction was performed to detect the H5 hemagglutinin gene. The set of oligonucleotides which performed best for the Nigerian samples was using primers 5’-GAG-AGG-AAA-TAA-GTG-GAG-TAA-AAT-TGG-A-3’, 5’- AAG-ATA-GAC-CAG-CTA-CCA-TGA-TTG-C-3’ and probe 5’6-FAM-TTT-ATT-CAACAG-TGG-CGA-GTT-CCC-TAG-CAC-T-TAMRA-3’. Virus isolation and characterization. For influenza A virus RT-PCR positive samples, 200 µl of the original material was inoculated into the allantoic cavity of 11-day-old embryonated hens’ eggs. The allantoic fluid was harvested two days after inoculation and influenza A virus was detected using hemagglutination assays with turkey red blood cells. Some virus isolates were obtained from inoculated MDCK cells. Sequencing and data analysis. PCR products were purified using the Jetquick PCR purification kit (Genomed, Loehne Germany). Purified products were quantified with Pico Green (Molecular Probes, Leiden, The Netherlands) using a Tecan Genios Reader (Mechelen, Belgium). 10 ng DNA were used for sequencing in both directions with the Big Dye Terminator v.3.1 cycle sequencing kit (Applied Biosystems, Nieuwerkerk, The Netherlands) on a capillary sequencer (model 3130 avant, Applied Biosystems, Nieuwerkerk, The Netherlands) using the PCR primers as sequencing primers. Phylogenetic and molecular evolutionary analyses were conducted using the Maximum-Likelihood method implemented in the PHYLIP Phylogeny Interference Package v 3.62 with an estimated transition/transversion ratio and the gamma distribution substitution model. Bootstrapping has been conducted with 100 replicates. Accession numbers of sequences in the phylogenetic tree (Fig. 1). A/chicken/Lagos.NIE/10.06/BA209(H5N1), AM262541 A/chicken/Lagos.NIE/10.06/BA210(H5N1), AM262542 A/chicken/Lagos.NIE/10.06/BA211(H5N1), AM262543 A/chicken/Lagos.NIE/8.06/SO300(H5N1), AM262546 A/chicken/Lagos.NIE/8.06/SO452(H5N1), AM262547 A/chicken/Lagos.NIE/8.06/SO493(H5N1), AM262553 A/chicken/Lagos.NIE/8.06/SO494(H5N1), AM262572 Supplementary figure S1. Origin of three lineages co-circulating in Nigeria. Inset: location of the affected SO and BA farms in Lagos state, Nigeria. Arrows indicate sites of avian influenza virus outbreaks in Nigeria for which sequences are available.