File
advertisement

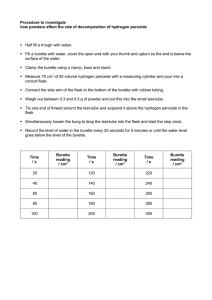
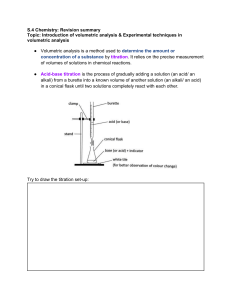
Unit 4: Scientific Practical Techniques Name: Jamie Gooding Assignment: 4.1 Analytical Techniques Describe the principles behind the technique, method or instrument you have used. For some synthesised chemical compounds - pharmaceuticals, for example - it is very important that they have a high level of purity. Just a tiny amount of an impurity in a drug could cause a great deal of harm to a patient. Samples of chemicals that are synthesised must be checked for purity. This is often done by carrying out a titration. A titration is used to measure the volume of one solution that exactly reacts with another solution. Steps of titration A titration is carried out using a number of steps: 1.If the sample is a solid, it is weighed using an accurate balance, and then dissolved to make up a known volume of solution (usually 100cm3). 2.A pipette is used to measure accurately a volume of this solution - for example, 10cm3. A safety pipette filler is used to draw solution into the pipette. This is emptied into a conical flask. 3.A few drops of an indicator may be added to the conical flask. This will show a change of colour when the titration is complete. 4.A second chemical is placed in a burette. This other solution is of a chemical that will react with the synthesised chemical sample in the conical flask. Often the solution in the burette is an acid or alkali, and it must be of a precise, known concentration. 5.The solution from the burette is run into the conical flask. The solution is added one drop at a time, with swirling to mix the solutions as the end-point is approached. Eventually, a colour change shows that the correct amount has been added to react completely with the synthesised chemical in the sample. 6.The volume of solution added from the burette is noted. The titration results can then be used to calculate the amount of the synthesised chemical in the sample, and therefore find its purity. Making Standard Solutions A standard solution is a method used to produce a solution that contains precisely a known concentration of an element or substance. Discuss why you used a volumetric flask when you made your sample and not a measuring cylinder. Describe any possible sources of error or contamination in the technique or process. Describe how these sources of error or contamination in the technique or process can be minimised. Used unopened samples of the chemical being used. Ensure that the balance is calibrated before use Check all glassware for faults before use. Ensure that the meniscus is read at eye level Check all calculations of RFM before starting Ensure all substances are stored in airtight containers to avoid absorption or loss of water from the air. Explain the possible consequences of the error or contamination. The standard solutions that are produced will not be at the desired concentrations and cannot therefore be used as our known concentrations. This would result in any titrations that are carried out with these solutions being incorrect. Suggest any improvements you could make to the technique, justifying your suggestions. Ensure that the equipment is washed out with distilled water when the dissolved solutions are transferred from beaker to volumetric flask. This will ensure that any undissolved substances are transferred to the volumetric flask making the standard solution more accurate. Describe the principles behind the technique, method or instrument you have used. This should include a brief outline of the scientific theory i.e. how does it work and why Performing a titration A titration is a method that is used to determine the unknown concentration of a reactant. An indicator is used to assess the end point of the reaction. Discuss why you used a glass pipette when measuring out your unknown concentration. Describe any possible sources of error or contamination in the technique or process You can make errors in the volume you deliver from your burette by the following means. 1) Your burette leaks (check the stopcock) 2) You incorrectly fill and/or read it due to parallax. (Parallax is an error that results when you look at the gradations on glassware from the wrong angle, or don't look at it from several angles. You visually misplace the meniscus and thus read it wrongly. 3). Bubbles in your burette or stopcock tip lower the volume actually present. 4). You may read the burette correctly but if you over- or undershoot your volumes (by inexperienced or incorrect use of the stopcock) you get errors that way. 5) Your sample volume is incorrect or inaccurate.
![BAI HAYYAT PAGLALA - [Template] [Template] Chem 1101B Exp. 4.](http://s1.studylib.net/store/data/025612661_1-90f80f72c7afd19687516e5c2a09b92e-300x300.png)