DNA extraction from whole blood by salt ppt
advertisement

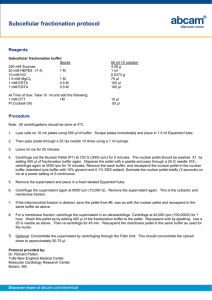
Court Lab / Kristina Cvitanovic Created April 2006 Salt Precipitation Method for DNA Extraction from Whole Blood - Adapted from method found at http://www.protocol-online.org Reagents Buffer A (Red blood cell lysis buffer) composition (for 250 ml solution) 0.32 M sucrose (27.38 g) 10 mM Tris HCl (0.394 g) 5 mM MgCl2 (0.254 g) 0.75% Triton-X-100 (1.875 ml) Adjust pH to 7.6 Buffer B (Proteinase K buffer) composition (for a 250 ml solution) 20 mM Tris-HCl (0.788 g) 4 mM Na2EDTA (0.372 g) 100 mM NaCl (1.461 g) Adjust pH to 7.4 *All solutions should be sterile (0.2μM filtered) Protocol: 1. Add 2 ml of Buffer A to 2 ml of whole blood and 2 ml of cold, sterile, deionised water. Invert tube 6-8 times and leave to incubate on ice for 2-3 minutes. 2. Centrifuge at 3500 rpm for 15 minutes. Discard supernatant into 2.5% bleach solution. Re-suspend pellet in 2 ml of Buffer A and 6 ml of water by vortexing for 30 seconds at medium speed. Centrifuge at 3500 rpm for 15 minutes and repeat washing step. 3. Add 5 ml of Buffer B and 500 μl of 10% SDS to pellet. Re-suspend pellet by vortexing vigorously for 30-60 seconds. Then add 50 μl of fresh, refrigerated Proteinase K solution (20 mg/ml). 4. Leave to incubate for 2 hours at 55˚C. Remove samples and leave for 2-3 min on ice to cool. Add 4 ml of 5.3 M NaCl (77.43 g for 250 ml solution). Vortex for 15 seconds. 5. Centrifuge samples at 4500 rpm for 20 minutes. Pour off supernatant into a fresh tube taking care not to dislodge the pellet. Add an equal volume of cold isopropanol (stored at - 20˚C). Invert 5-6 times gently to precipitate DNA. 6. Remove DNA with a wide bore tip and transfer to a microcentrifuge tube. Wash with 1 ml of 70% ethanol. Leave DNA to dry at room temperature. Re-suspend DNA in 300 μl of water. Allow DNA to re-dissolve overnight at room temperature.