Links - Exordio
advertisement

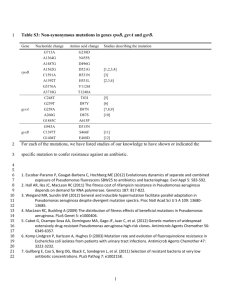
12 October 2000 Nature 407, 762 - 764 (2000) © Macmillan Publishers Ltd. <> Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms PRADEEP K. SINGH*†, AMY L. SCHAEFER†‡, MATTHEW R. PARSEK†§, THOMAS O. MONINGER , MICHAEL J. WELSH* & E. P. GREENBERG‡ * Howard Hughes Medical Institute & Department of Internal Medicine, University of Iowa College of Medicine , Iowa City, Iowa 52242, USA ‡ Department of Microbiology, University of Iowa College of Medicine, Iowa City, Iowa 52242, USA § Department of Civil Engineering, Northwestern University, Evanston, Illinois 60208, USA Central Microscopy Research Facility, University of Iowa College of Medicine, Iowa City, Iowa 52242, USA † These authors contributed equally to this work Correspondence and requests for materials should be addressed to E.P.G. (e-mail: everett-greenberg@uiowa.edu). The bacterium Pseudomonas aeruginosa permanently colonizes cystic fibrosis lungs despite aggressive antibiotic treatment1-3. This suggests that P. aeruginosa might exist as biofilms—structured communities of bacteria encased in a self-produced polymeric matrix—in the cystic fibrosis lung1, 4. Consistent with this hypothesis, microscopy of cystic fibrosis sputum shows that P. aeruginosa are in biofilm-like structures. P. aeruginosa uses extracellular quorum-sensing signals (extracellular chemical signals that cue cell-density-dependent gene expression) to coordinate biofilm formation5. Here we found that cystic fibrosis sputum produces the two principal P. aeruginosa quorumsensing signals; however, the relative abundance of these signals was opposite to that of the standard P. aeruginosa strain PAO1 in laboratory broth culture. When P. aeruginosa sputum isolates were grown in broth, some showed quorum-sensing signal ratios like those of the laboratory strain. When we grew these isolates and PAO1 in a laboratory biofilm model, the signal ratios were like those in cystic fibrosis sputum. Our data support the hypothesis that P. aeruginosa are in a biofilm in cystic fibrosis sputum. Moreover, quorum-sensing signal profiling of specific P. aeruginosa strains may serve as a biomarker in screens to identify agents that interfere with biofilm development. Pseudomonas aeruginosa is the principal pathogen in the lungs of patients with cystic fibrosis (CF). Chronic colonization by this bacterium leads to progressive lung damage, and eventually respiratory failure and death in most CF patients2, 3, 6. Once the CF lung has been colonized, even long-term antibiotic therapy does not eradicate P. aeruginosa2, 3, 7 . One hypothesis for the antibiotic resistance of P. aeruginosa in the CF lung is that this bacterium exists there as a biofilm, as bacterial biofilms are innately resistant to antimicrobial treatment1. Because little is known about the physiology of P. aeruginosa cells in biofilms, the hypothesis that P. aeruginosa in the CF lung is a biofilm has not been rigorously tested1, and a marker for the biofilm-state has not been identified. Here, we first used transmission electron microscopy to examine CF sputum from patients infected with only P. aeruginosa . We prepared samples using a perflurocarbon-based protocol to avoid the extraction of water-soluble material inherent in aqueous-based methods. We observed clusters of P. aeruginosa encased in a densely stained matrix (Fig. 1). Bacterial clusters were easily distinguished from the surrounding heterogeneous material containing host cells. We examined sputum samples from three different patients, and we did not observe bacteria outside these structures. This appearance is consistent with previous microscopic observations and consistent with the hypothesis that in CF sputum, P. aeruginosa exists in biofilms. As microscopic observations are open to interpretation, however, we sought physiological evidence of a biofilm lifestyle. Figure 1 Transmission electron microscope images of P. aeruginosa in sputum. Full legend High resolution image and legend (148k) Biofilm development involves several steps. After attachment and multiplication on a surface, bacterial microcolonies form and then differentiate into characteristic antibiotic-resistant structures with cells encased in an extracellular polysaccharide matrix. In P. aeruginosa, differentiation is cued by one of two quorum-sensing signals that control the expression of dozens of P. aeruginosa genes in a cell-density-dependent fashion8, 9. The signals are N-(3-oxododecanoyl)-L-homoserine lactone (3OC12-HSL) and N-butyryl-L-HSL (C4-HSL). The signal required for microcolony differentiation in biofilms is 3OC12-HSL5). Although studies with mice indicate that quorum sensing is important for virulence of P. aeruginosa10, 11, it has not been shown that acyl-HSLs are produced by P. aeruginosa in lung secretions from CF patients or from any other human samples. This is due to limitations of the bioassays normally used to detect acyl-HSLs12. We have developed a radiometric technique that allows us to measure relative synthesis rates of acyl-HSLs in biological samples13. Using this technique, we showed that broth cultures of the common laboratory strain P. aeruginosa PAO1 produce 3OC12-HSL at a rate between 3 and 10 times that of C4-HSL production (Fig. 2). We then used the radiometric technique to test the hypothesis that P. aeruginosa in CF sputum produces acyl-HSL signals. In all cases, sputum samples from patients colonized with P. aeruginosa produced acyl-HSLs, but, in contrast to the broth-grown cultures of P. aeruginosa PAO1, the sputum samples produced more C4-HSL than 3OC12-HSL (Fig. 2). Clinical microbiology results showed that some sputum samples contained mucoid strains of P. aeruginosa that often colonize CF patients, others contained nonmucoid strains or more then one strain, and some sputum samples contained bacterial species in addition to P. aeruginosa ( Table 1). We did not detect acyl-HSL synthesis in sputum from two CF patients free of P. aeruginosa but colonized by Staphylococcus aureus. Figure 2 Acyl-HSL profiles of P. aeruginosa broth cultures and CF sputum samples. Full legend High resolution image and legend (29k) The differences in C4-HSL to 3OC12-HSL ratios in sputum versus pure cultures grown in laboratory broth might relate to the different conditions in which P. aeruginosa is growing or it might be due to strain variation. To discriminate between these two possibilities, we analysed laboratory-grown broth cultures of nine clinical isolates from six of the sputum samples. Of the nine isolates, three produced more 3OC12-HSL than C4-HSL in culture broth ( Fig. 3b). This finding indicates that for these isolates the conditions determined the ratio of acyl-HSLs. The other six isolates produced more C4HSL than 3OC12-HSL when cultured in laboratory broth. In one isolate another acylHSL, C6-HSL, was the most abundant signal molecule. We also tested several non-CF clinical isolates of P. aeruginosa, and two P. aeruginosa environmental isolates, one from sewage and the other from a hot tub. All of these isolates produced more C4-HSL than 3OC12-HSL in laboratory broth (data not shown). Figure 3 Comparisons of acyl-HSL ratios in biofilms with those in broth cultures. Full legend High resolution image and legend (55k) Two of the three CF clinical isolates for which the environmental conditions determined whether the predominant acyl-HSL was C4-HSL or 3OC12-HSL came from sputum in which only one type of P. aeruginosa was isolated. The third isolate was the predominant type (the mucoid type, at 100-fold higher levels then the nonmucoid type) in the sputum from which it was isolated. As a test of the hypothesis that P. aeruginosa exists as a biofilm in CF lungs1, we asked whether the strains that produced predominantly 3OC12-HSL in broth cultures would produce sputum-like acyl-HSL ratios when grown as biofilms. We established biofilms in silicone tubing (see Methods; and Fig. 3a), and measured C4-HSL:3OC12-HSL ratios (Fig. 3b). The acyl-HSL ratios produced by the biofilms showed the pattern characteristic of sputum. Thus, under the conditions of our experiments, the acyl-HSL profiles of these strains serve as a biomarker of their growth as a biofilm. This analysis of the three biomarker strains isolated from CF sputum provides evidence that in CF sputum P. aeruginosa exists primarily as a biofilm. These strains produce C4-HSL in excess of 3OC12-HSL when grown in biofilms but not when grown in broth culture. The sputum from which they were isolated showed acyl-HSL profiles like the biofilms. This would not be expected if only a small portion of the bacteria in the sputum existed as a biofilm. Our findings are consistent with the hypothesis that P. aeruginosa in CF lungs exist as a biofilm. However, previous evidence in support of this hypothesis is scant1, 14, 15, and the hypothesis has not addressed the question of whether a large or small percentage of the bacterial cells in a CF lung infection exists as a biofilm. The radiometric technique allowed us to show that P. aeruginosa in CF sputum generates quorum-sensing signals, and allowed the identification of strains that show acyl-HSL profiles indicative of growth in a biofilm state. One might imagine that under different conditions either biofilms or routine laboratory cultures could exhibit different acyl-HSL profiles than the ones we observed. Nevertheless, with defined conditions the indicator strains should be useful in screening efforts aimed at identification of agents that block or disrupt normal biofilm activity. Methods Sputum samples Sputum (2–10 ml) was obtained from CF patients attending the University of Iowa CF Clinic for routine follow-up or with exacerbation of CF lung disease. Sputum was collected by voluntary expectoration. Patients gave informed consent and the protocol was approved by the University of Iowa Human Subjects Review Board. One millilitre of each sample was analysed at the University of Iowa Diagnostic Laboratory to determine the species and numbers of bacterial colonyforming units present. Microscopic examination was performed on freshly expectorated sputum from three CF patients colonized only with P. aeruginosa. Osmium tetroxide (1%) was dissolved in perfluorocarbon (Fluorinert FC-72, 3M Corp.). Samples were gently immersed in the perfluorocarbon fixative for 1 h followed by three rinses in pure perfluorocarbon. Samples were then dehydrated in three washes of 100% ethanol, transitioned to Eponate-12 resin (Ted Pella Inc., CA) and cured overnight at 65 °C. Ultrathin sections were stained with uranyl acetate and lead citrate and imaged in a Hitachi H-7000 transmission electron microscope. Analysis of acyl-HSL production For analysis of acyl-HSL production in sputum, fresh samples were homogenized by vortexing with glass beads in an equal volume of PBS containing 2% N-acetylcysteine. The sputum was labelled with 10 µCi of 14Ccarboxy-methionine (American Radiochemical Company) for 4 h with shaking at 37 °C. The acyl-HSLs were extracted with two equal volumes of acidified ethyl acetate (100 µl glacial acetic acid per litre solvent). The solvent was removed by evaporation under a gentle stream of N2 gas, and the residue was suspended in 200 µl of 50% methanol in water and separated by methanol-gradient C18 reverse phase high performance liquid chromatography (HPLC) (10–100% methanol) as described16. We determined radioactivity by scintillation counting. Corrections were made on the basis of the extraction coefficients of the acyl-HSLs. The acyl-HSL assignments were made by comparison with retention times of synthetic acyl-HSLs. The 4-h incubation period and the amount of radiolabel used were determined empirically to give sufficient product in our samples for ease of analysis. With shorter incubation periods (1 h) and less 14Ccarboxy-methionine (5 µCi), there was more noise in the HPLC profiles but the C4HSL:3OC12-HSL ratios were similar to those shown in Fig. 2. For analysis of acyl-HSL production in broth cultures, we grew all strains of P. aeruginosa in Jensen's Medium with 0.3% glycerol at 37 °C with shaking17. Unless otherwise indicated, we added 5 µCi of 14C-carboxy-methionine to cultures at an absorbance at 600 nm (A600) of 1. After 10 min, we extracted and analysed the acylHSLs as described above. Biofilms We grew biofilms at 37 °C in silicone tubing (3.2 mm diameter) using a oncethrough continuous-flow system18. Jensen's Medium with 0.3% glycerol was pumped through a 19-cm section of tubing that had an internal volume of 1 ml at a rate of 0.2 ml min-1. The tubing was inoculated with 1 ml of an overnight broth culture of P. aeruginosa by syringe, and after 15 min the flow of medium was initiated. To analyse acyl-HSL production, we halted flow and injected 10 µCi of 14C-carboxy-methionine in 1 ml of pre-warmed medium into the tube. After 30 min, we analysed the contents of the silicone tube as described above. Samples were prepared for scanning electron microscopy by fixation in 2.5% gluteraldehyde followed by 1% osmium, dehydration in ethanol followed by hexamethyldisilizane, and air drying. The dried samples were mounted on aluminium stubs, sputter-coated with gold and palladium (60:40), and imaged with a Hitachi S4000 electron microscope. Differences in incubation time and in the amount of 14C-carboxy-methionine added to broth cultures and laboratory biofilms of P. aeruginosa PAO1 did not appear to influence C4-HSL:3OC12-HSL ratios. With both broth cultures and laboratory biofilms, we carried out experiments with 10 µCi of 14C-carboxy-methionine and with incubation times of up to 1 h; the acyl-HSL ratios were indistinguishable from those we report here. Furthermore, under a variety of growth conditions (different carbon sources and anaerobic growth) broth cultures of P. aeruginosa PAO1 produced 3OC12-HSL in excess of C4-HSL. Laboratory biofilms of P. aeruginosa PAO1 always produced more C4-HSL than 3OC12-HSL, regardless of incubation time (24–100 h) (M.R.P., A.L.S. and E.P.G., manuscript in preparation). Received 17 July 2000; accepted 8 August 2000 References 1. Costerton, J. W., Stewart, P. S. & Greenberg, E. P. Bacterial biofilms: a common cause of persistant infections. Science 284, 1318-1322 (1999). Links 2. Burns, J. L., Ramsey, B. W. & Smith, A. L. Clinical manifestations and treatment of pulmonary infections in cystic fibrosis. Adv. Pediatr. Infect. Dis. 8, 53-66 (1993). Links 3. Hoiby, N. Antibiotic therapy for chronic infection of Pseudomonas in the lung. Annu. Rev. Med. 44, 1-10 (1993). Links 4. Costerton, J. W., Lewandowski, Z., Caldwell, D. E., Korber, D. R. & Lappin-Scott, H. M. Microbial biofilms. Annu. Rev. Microbiol. 49, 711-745 (1995). Links 5. Davies, D. G. et al. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science 280, 295-298 (1998). Links 6. Welsh, M. J., Tsui, L.-C., Boat, T. F. & Beaudet, A. L. in Metabolic and Molecular Basis of Inherited Diseases Vol. III (eds Scriver, C. R., Beaudet, A. L., Sly, W. S. & Valle, D.) 37993876 (McGraw-Hill, New York, 1995). 7. Mendelman, P. et al. Aminoglycoside penetration, inactivation, and efficacy in cystic fibrosis sputum. Am. Rev. Respir. Dis. 132, 761-765 (1985). Links 8. Whiteley, M., Lee, K. M. & Greenberg, E. P. Identification of genes controlled by quorum sensing in Pseudomonas aeruginosa. Proc. Natl Acad. Sci. USA 96, 13904-13909 (1999). Links 9. Pesci, E. C. & Iglewski, B. H. The chain of command in Pseudomonas quorum sensing. Trends Microbiol. 5, 132-135 (1997). Links 10. Tang, H. B. et al. Contribution of specific Pseudomonas aeruginosa virulence factors to pathogenesis of pneumonia in a neonatal mouse model of infection. Infect. Immun. 64, 3743 (1996). Links 11. Rumbaugh, K. P., Griswold, J. A. & Hamood, A. N. Contribution of the regulatory gene lasR to the pathogenesis of Pseudomonas aeruginosa infection of burned mice. J. Burn Care Rehabil. 20, 42-49 (1999). Links 12. Schaefer, A. L., Hanzelka, B. L., Parsek, M. R. & Greenberg, E. P. Detection, purification and structural elucidation of acylhomoserine lactone inducer of Vibrio fischeri luminescence and other related molecules. Methods Enzymol. 305, 288-301 (2000). Links 13. Schaefer, A. L., Greenberg, E. P. & Parsek, M. R. Methods Enzymol. (in the press). 14. Costerton, J. W. The etiology and persistence of cryptic bacterial infections: a hypothesis. Rev. Infect. Dis. 6, S608-S616 (1984). Links 15. Costerton, J. W., Lam, J., Lam, K. & Chan, R. The role of the microcolony mode of growth in the pathogenesis of Pseudomonas aeruginosa infections. Rev. Infect. Dis. 5, S867-S873 (1983). Links 16. Pearson, J. P. et al. Structure of the autoinducer required for expression of Pseudomonas aeruginosa virulence genes. Proc. Natl Acad. Sci. USA 91, 197-201 (1994). Links 17. Jensen, J. E., Fecycz, T. & Campell, J. N. Nutritional factors controlling exocellular protease production by Pseudomonas aeruginosa. J. Bacteriol. 144, 844-847 (1980). Links 18. Davies, D. G., Chakrabarty, A. M. & Geesey, G. G. Exopolysaccharide production in biofilms: substratum activation of alginate gene expression by Pseudomonas aeruginosa. Applied Environ. Microbiol. 59, 1181-1186 (1993). Acknowledgements. We thank J. Launspach, C. Clark, and the University of Iowa Clinical Microbiology Laboratory for providing the data in Table 1. Funding was provided by the National Insititute of General Medical Sciences, the National Heart, Lung, and Blood Institute, and the Cystic Fibrosis Foundation. A.L.S. was a NIH Graduate Trainee, M.R.P. was the recipient of an NIH Postdoctoral Research Service Award, and P.K.S. is the recipient of an NIH Mentored Physician Scientist Award and a Cystic Fibrosis Foundation Leroy Matthews Award. Figure 1 Transmission electron microscope images of P. aeruginosa in sputum. a, Low magnification; b, high magnification. P. aeruginosa are organized in clusters encased in a densely staining matrix. Scale bars, 1 µm. Figure 2 Acyl-HSL profiles of P. aeruginosa broth cultures and CF sputum samples. a, Examples of P. aeruginosa (top) and sputum (bottom) HPLC profiles. C4-HSL elutes in fractions 10–11; 3OC12-HSL elutes in fractions 57–61. The analysis of P. aeruginosa PAO1 was with a midlogarithmic phase culture. b, C4-HSL as a fraction of the total C4-HSL plus 3OC12-HSL. From left to right, P. aeruginosa in early logarithmic (A600 = 0.5), mid-logarithmic (A600 = 1), late logarithmic (A600 = 2), stationary (A 600 = 4) and late stationary phase (A600 = 6). Sputum samples were analysed as described in Methods. Three samples have been lettered because the predominant P. aeruginosa isolates from these samples have been subjected to a more extensive analysis. Figure 3 Comparisons of acyl-HSL ratios in biofilms with those in broth cultures. a, Scanning electron micrograph of a P. aeruginosa PAO1 biofilm in a silicone tube. The tube was sliced open, and the sample was prepared for scanning electron microscopy. Microcolonies can be seen. Scale bar, 10 µm. b, C4-HSL as a fraction of the total C4-HSL plus C12-HSL in broth cultures (filled squares) and biofilms (open squares) of P. aeruginosa PAO1, and the most abundant bacterial type in sputum samples A, B and C (see Table 1). Data are mean s.d. for PAO1; broth and biofilm ratios were significantly different (P < 0.001). Ratios for CF isolates studied under different conditions were also statistically different (P < 0.005).