APPLICATION - Department of Agriculture and Rural Development
advertisement
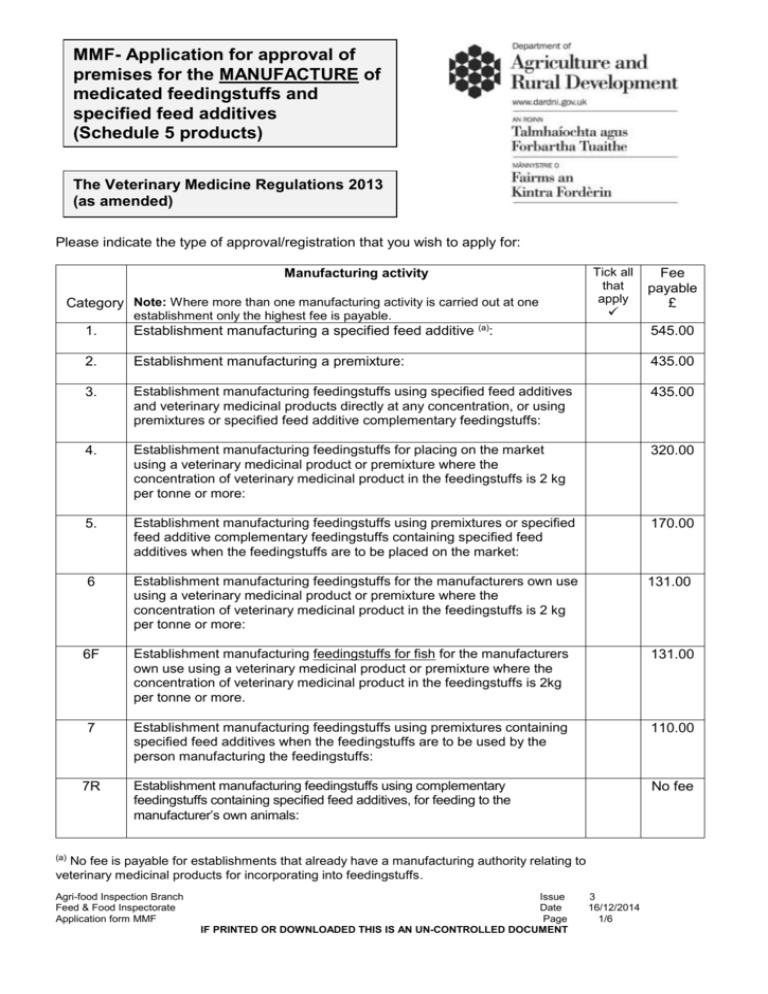
MMF- Application for approval of premises for the MANUFACTURE of medicated feedingstuffs and specified feed additives (Schedule 5 products) The Veterinary Medicine Regulations 2013 (as amended) Please indicate the type of approval/registration that you wish to apply for: Manufacturing activity Category Note: Where more than one manufacturing activity is carried out at one establishment only the highest fee is payable. Tick all that apply Fee payable £ 1. Establishment manufacturing a specified feed additive (a): 545.00 2. Establishment manufacturing a premixture: 435.00 3. Establishment manufacturing feedingstuffs using specified feed additives and veterinary medicinal products directly at any concentration, or using premixtures or specified feed additive complementary feedingstuffs: 435.00 4. Establishment manufacturing feedingstuffs for placing on the market using a veterinary medicinal product or premixture where the concentration of veterinary medicinal product in the feedingstuffs is 2 kg per tonne or more: 320.00 5. Establishment manufacturing feedingstuffs using premixtures or specified feed additive complementary feedingstuffs containing specified feed additives when the feedingstuffs are to be placed on the market: 170.00 6 Establishment manufacturing feedingstuffs for the manufacturers own use using a veterinary medicinal product or premixture where the concentration of veterinary medicinal product in the feedingstuffs is 2 kg per tonne or more: 131.00 6F Establishment manufacturing feedingstuffs for fish for the manufacturers own use using a veterinary medicinal product or premixture where the concentration of veterinary medicinal product in the feedingstuffs is 2kg per tonne or more. 131.00 7 Establishment manufacturing feedingstuffs using premixtures containing specified feed additives when the feedingstuffs are to be used by the person manufacturing the feedingstuffs: 110.00 Establishment manufacturing feedingstuffs using complementary feedingstuffs containing specified feed additives, for feeding to the manufacturer’s own animals: No fee 7R (a) No fee is payable for establishments that already have a manufacturing authority relating to veterinary medicinal products for incorporating into feedingstuffs. Agri-food Inspection Branch Feed & Food Inspectorate Application form MMF Issue Date Page IF PRINTED OR DOWNLOADED THIS IS AN UN-CONTROLLED DOCUMENT 3 16/12/2014 1/6 THIS IS NOT AN INVOICE – DO NOT SEND PAYMENT WITH THIS APPLICATION FORM Section 1 – Applicant’s Details Section 2 – Premises Details (where activity is carried out) Applicant’s name: Trading Name (if different form applicant’s/corporate body or company name) Corporate body/Company Name Address (if different from applicant’s address) Address: Postcode: Postcode: Tel. No: Tel. No: Mob. No: Mob. No: Fax No: Fax No: E-mail address: E-mail address: Website: Website: Additional information eg opening hours, specific biosecurity measures: Species of animals kept (where applicable): *Cattle / Sheep / Pigs / Poultry / Other (give details): *delete as applicable What mixing equipment is used? Wet-feeding system? (if applicable) Agri-food Inspection Branch Feed & Food Inspectorate Application form MMF Yes No Issue Date Page IF PRINTED OR DOWNLOADED THIS IS AN UN-CONTROLLED DOCUMENT 3 16/12/2014 2/6 Section 3 – Designated Persons (Responsible for Production) Title: Dr / Mr / Mrs / Miss / Ms (delete where applicable) Title: Dr / Mr / Mrs / Miss / Ms (delete where applicable) First name: First name: Surname: Surname: Tel. No: Tel. No: Mob. No: Mob. No: Fax No: Fax No: E-mail address: E-mail address: Section 4 – Declaration Please Note: A separate application form must be submitted for each premises for which approval or registration is required. Declaration I confirm that I have read the requirements for approval/registration as a manufacturer of Schedule 5 products under the current Veterinary Medicines Regulations, and hereby declare that the said premises, equipment and procedures meet the requirements [1] and are ready for inspection. Please note that once an application has been received and an approval inspection carried out, fees are not refundable. Signed: ________________________________________________ Print Name: _ Date: ___ ____________________________________________ ______________ Position in Company: __ Agri-food Inspection Branch Feed & Food Inspectorate Application form MMF ____________________________________ Issue Date Page IF PRINTED OR DOWNLOADED THIS IS AN UN-CONTROLLED DOCUMENT 3 16/12/2014 3/6 DARD will use the information you have compulsorily provided to fulfil its statutory functions, which includes publishing the premises name & address in the Register of Approved/Registered Feedingstuffs Manufacturers & Distributors. Otherwise, the information will not be disclosed to third parties without your consent. The information held is subject to current data protection legislation. The summary of requirements for premises approval and registration can be found in Veterinary Medicines Guidance Note No.17 – Medicated Feedingstuffs and Specified Feed Additives on the VMD website: www.vmd.defra.gov.uk or by contacting Agri-food Inspection Branch, DARD on Tel: (028) 9076 5868. Farmers manufacturing feedingstuffs using veterinary medicinal products, or premixtures containing veterinary medicinal products or specified feed additives, should note the attached checklist. Please do NOT send any payment now as an invoice from our accounts branch will follow on receipt of your application form. Section 5 - Return of Application Form Please return completed application form to: Department of Agriculture and Rural Development Agri-food Inspection Branch Room 1018, Dundonald House Upper Newtownards Road Dundonald Belfast BT4 3SB. Enquiries to: Tel: (028) 9076 5868 Tel: (028) 9076 5379 Fax: (028) 9052 4671 E-mail: afib.admin@dardni.gov.uk Agri-food Inspection Branch Feed & Food Inspectorate Application form MMF Issue Date Page IF PRINTED OR DOWNLOADED THIS IS AN UN-CONTROLLED DOCUMENT 3 16/12/2014 4/6 Checklist for farmers mixing animal feedingstuffs containing Controlled Products Controlled Products In this checklist, Controlled Products refer to: • Veterinary Medicinal Products (VMPs) authorised for mixing into animal feed; • Specified Feed Additives (SFAs) i.e. coccidiostats and growth promoters; and • Premixtures containing VMPs or SFAs. Premises & Equipment Ensure that all parts of the premises and equipment used for mixing feeds, including associated storage areas, are: In a good state of repair. Clean and tidy. Free from spillages, old packaging and obsolete equipment. Protected from the entrance and harbouring of pests, vermin and domestic animals. Well lit, ventilated and dry. Suitable to accurately mix feeds and protect feeds from contamination. Personnel Ensure that: There is a designated person responsible for feed production. Staff are trained and knowledgeable in handling Controlled Products and mixing feeds. Appropriate staff facilities and personal protective equipment are available. Production Ensure that procedures are in place so: Feeds are mixed according to written formulations and instructions aimed at minimising crosscontamination during mixing, storage and transport. Veterinary medicines are only used in accordance with a valid Medicated Feedingstuff (MFS) prescription. Other Controlled Products are only used ‘as labelled’. Waste material not suitable for feed is isolated and identified for disposal. Storage & Transport Ensure that: Controlled Products are stored in their original packaging and in a secure location. Storage areas for feed materials and mixed feed are suitable to prevent contamination and pest damage. All feed materials and mixed feeds are labelled or otherwise clearly identifiable. Mixed feeds are transported in suitable vehicles or containers, which are cleaned as necessary to prevent cross-contamination. Agri-food Inspection Branch Feed & Food Inspectorate Application form MMF Issue Date Page IF PRINTED OR DOWNLOADED THIS IS AN UN-CONTROLLED DOCUMENT 3 16/12/2014 5/6 Quality Control Ensure that a Quality Control plan is drawn up which includes: Keeping samples of mixed feeds. Testing the efficiency of the mixer (homogeneity test). Testing samples of mixed feed for Controlled Products and other substances. Testing mixed feed for cross-contamination (carryover) where there is a risk. Monitoring feed materials and mixed feeds for harmful organisms and deleterious substances. Record-keeping Ensure that appropriate records are kept of the following: Controlled Products and feed materials purchased. Feeds mixed and details of the Controlled Products used (including batch numbers). MFS prescriptions to cover purchase and use of VMPs and medicated premixtures. Documented Procedures for feed production. Quality Control results: - mixer efficiency (homogeneity) tests - carryover (cross-contamination) tests - calibration of weighing/measuring equipment - analysis of mixed feeds and feed materials Cleaning and maintenance of premises, storage areas and equipment. HACCP plan. All records are kept for 5 years. Further guidance is available from: Department of Agriculture and Rural Development Agri-food Inspection Branch Room 1018 Dundonald House Upper Newtownards Road Ballymiscaw Dundonald Belfast BT4 3SB. Tel: (028) 9076 5868 E-mail :lorraine.colgan@dardni.gov.uk E-mail: gerard.higgins@dardni.gov.uk Agri-food Inspection Branch Feed & Food Inspectorate Application form MMF Issue Date Page IF PRINTED OR DOWNLOADED THIS IS AN UN-CONTROLLED DOCUMENT 3 16/12/2014 6/6







