Thermoelectricity Introduction You may be aware that a

Thermoelectricity
Introduction
You may be aware that a thermocouple is a transducer that converts a temperature difference into an emf.
It consists of two wires of dissimilar metals: a common combination is copper-constantan
1
. The thermocouple's use as a thermometer is widespread because it is easily constructed and its small size does not perturb the thermal properties of small samples.
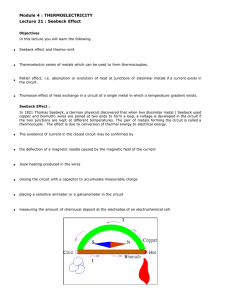
Less well known is that when current flows through a thermocouple, heat is liberated at one junction and absorbed at the other. This is known as the Peltier effect . In this experiment a thermocouple is used to estimate the performance of a Peltier refrigerator. The freezing curve of water can be measured as well as the effect of adding salt to water can be investigated.
When a temperature gradient is established across a conductor the free carriers at the hot end have more kinetic energy thereby tending to diffuse towards the cold end. As this occurs a heat current
.
Q , carried by the diffusing electrons, flows from the hot to cold end. As this heat current is flowing an electric field develops which tends to oppose the heat flow. Thus an emf appears along the conductor which is proportional to the temperature difference:
V
S
T .
(1)
This is referred to as the Seebeck effect where S is the Seebeck coefficient.
Before considering an actual thermocouple let us consider a conductor made of some material A . If we connect a voltmeter with, say, copper leads across the conductor we will measure a voltage
V
( S
A
S
Cu
)
T
S
ACu
T .
(2)
Note that if material A is also Cu, the voltage will be zero.
1 Constantan is an alloy of Cu and Ni.
It is not necessary to use Cu in one leg of the thermocouple. Consider a thermocouple consisting of two materials A and B .
Convince yourself that the B-Cu and Cu-B emfs will cancel so long as both B-Cu junctions are at the same temperature - even if this isn't the temperature of the voltmeter.
Thus the net effect is just an AB thermocouple with
V
S
AB
( T
1
T
2
).
(3)
Calibration tables exist for common thermocouple materials. The selection of thermocouple material is governed by the operational temperature range as well as the local environment the materials are exposed to.
The cooling power of the Peltier refrigerator also depends on the two materials of the junction. If the current flowing across a junction of materials A and B is I then the rate of heat absorption is
.
Q
P
AB
I (4) where
AB is the Peltier coefficient at the junction of materials A and B . The Peltier coefficient is related to the Seebeck coefficient of the junction by
AB
TS
AB
(5) where T is the junction temperature. Note that the cooling power is proportional to I which is quite different from Joule heating which depends on
2
I .
You might note that the relation between the two coefficients in equation (5) is similar to the relation between the latent heat L associated with a phase change. Here
L
T
S where
S is the entropy change between the two phases. In the case of the
Peltier refrigerator the Seebeck coefficient is the rate of entropy moving from A to B .
This analogy is the base of the thermodynamics of this effect, due to Kelvin.
Semiconductors show much larger thermoelectric effects than do metals. Since semiconductors can't be drawn out into wire their use as thermocouples is limited, but an effective refrigerator can be made using blocks of semiconducting material as illustrated below. The materials used in the experiment are n and p doped bismuth telluride.
The higher electrical resistance of semiconductors results in more Joule heating which degrades the performance, but on the other hand the thermal resistance is also higher so the effect of direct conduction of heat is reduced.
Experiment
The main component of the apparatus is shown below with several of the key features labeled. Pay close attention to the wiring. You will need to connect current shunts to be able to measure both the current flowing through the Peltier refrigerator as well as the heaters.
The cooling fan and Peltier refrigerator start as soon as you plug the unit in. The DC current for the Peltier junction is supplied by the rectifier diodes mounted on the heat sink
below the power transformers. The heaters can be powered by a variable transformer with a step-down transformer.
In order to measure temperature you will need to make a copper-constantan thermocouple. The Cu and constantan wire and the necessary solder and soldering iron are available from the Resource Centre, MP229. Make sure the wire length is long enough to bridge the distance between the two temperature measuring tubes. This type of junction yields a thermal emf of
40
V/K near room temperature, which is a typical sensitivity for thermocouples.
Find the ratio of the Peltier junction thermoelectric potential to that of the Cuconstantan thermoelectric potential for temperatures near room temperature. This will allow you to determine the Seebeck coefficient of the Peltier junction given the Cuconstantan coefficient above.
Operate the Peltier refrigerator and measure the Peltier coefficient directly. In order to decide what operating conditions will lead to the best result, you should consider where heat flows if there is a temperature gradient across the Peltier junction. Sketch a heat flow diagram to aid in this process. What value of temperature gradient across the Peltier junction will lead to the least extraneous heat flow for this measurement?
Using equation (5) calculate the expected value of the Peltier coefficient and compare to the value measured directly.
Freeze a sample of water in the glass tube, monitoring the temperature of the water with your thermocouple. Track the temperature with time. Knowing the cooling power of the Peltier refrigerator you can estimate the latent heat associated with freezing. Note that under these conditions you will need to estimate how much heat is extracted from the water vs. the environment.
Add salt to the water sufficient to saturate the solution. Repeat the above step and comment on the difference in the result.
Reference
Theory of Thermoelectric Engineering - Instruction Manual
(The Ealing Corporation) - quite useful for this experiment; see Resource Centre.
GMG 82
DH 89
BWS 01