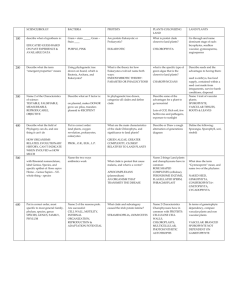
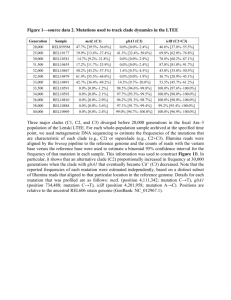
jbi12040-sup-AppendixS3
advertisement

1 SUPPORTING INFORMATION Delving into Delias Hübner (Lepidoptera: Pieridae): fine-scale biogeography, phylogenetics and systematics of the world’s largest butterfly genus Chris J. Müller, Pável F. Matos-Maraví and Luciano B. Beheregaray Journal of Biogeography Appendix S3 Delias systematics. Taxonomic appraisal of Delias The comprehensive taxon sampling in this study resolved 14 distinct clades. Both the number of clades and their fine-scale composition differ from that of Talbot (1928– 1937), Yagishita et al. (1993) and Braby & Pierce (2007). Nonetheless, the broadscale phylogeny of the latter authors does not differ markedly from our own. Clade relationships and composition within the new phylogenetic hypothesis was markedly constant irrespective of the analysis [i.e. maximum parsimony (MP) or Bayesian inference (BI)]. Our nysa clade corresponds to the singhapura clade of Braby & Pierce (2007), which included the singhapura, nysa and georgina species groups of earlier authors (Talbot, 1928–1937; Yagishita et al., 1993) and the brandti species group of Müller (2001). Yagishita et al. (1993) separated the georgina group from the nysa group but this is probably not warranted as D. nuydaorum Schröder and D. paoaensis Inomata & Nakano (from the ‘georgina’ group of Yagishita et al., 1993) are both nested within the ‘nysa’ complex in our analysis. The finer-scale relationships of the nysa clade revealed in this study are in major disagreement to previous authors. The clade comprises two main groups, with D. hempeli Dannatt, D. enniana Oberthür and D. vidua Joicey & Talbot [previously assigned to the nysa as opposed to the singaphura group by both Talbot (1928–1937) and Yagishita et al. (1993)] grouping with the singaphura group. We agree with species level status for members of Yagishita’s georgina group. Those that were known at the time were all included as subspecies 2 of D. georgina Felder & Felder by Talbot (1928–1935) but since the publication of his monograph sympatry between certain species has been recognized. We also concur with Braby & Pierce (2007), who implied that D. nysa (Fabricius) and D. brandti Müller are closely related. However, D. pulla and D. nysa were recovered as sister species, with D. brandti sister to that pairing. The isse clade also includes the dorimene clade and contains a mix of the previously recognised species groups. Braby & Pierce (2007) made a similar inference but included both with the hyparete species group in the isse clade, while in our phylogeny the isse clade is sister to the nysa clade. Delias laknekei Miller, Simon & Wills, a recently described species from New Ireland, Bismarck Archipelago, is sister to D. lytaea (Godman & Salvin) from proximal New Britain. This relationship suggests that the two species are allopatric and that D. lytaea is not likely to occur on New Ireland, as was previously supposed (e.g. D’Abrera, 1971, 1977, 1990). Indeed, the few records of D. lytaea from this island are dubious (Miller et al., 2007). Particularly noteworthy in our phylogeny is the phylogenetic position of D. chrysomelaena in the isse group, transferred from the ladas group (previously referred to as the chrysomelaena group). We found that the composition of the morphologically divergent ladas group, sampled entirely, is otherwise largely in agreement with that proposed by Talbot. For the most part, the pasithoe group forms an obvious grouping, as suggested by previous authors (Talbot, 1928–1937; Yagishita et al., 1993), and is corroborated in this study. Our phylogeny confirms that the distinctive D. benasu Martin, transferred from the belladonna group by Morishita (1981), belongs in the pasithoe group. Talbot (1928–1937) considered that D. benasu may be a link between the pasithoe and belladonna groups but in our analyses D. benasu is sister to D. woodi Talbot (also pasithoe group). Interestingly, D. blanca, transferred from the nysa to the belladonna group by Yata (1985) is actually nested within the pasithoe group. The belladonna group is sister to the hyparete clade and to the pasithoe clade, in our MP and Bayesian analyses, respectively. Delias aganippe Donovan is a particularly distinctive species that was provisionally placed, together with the pasithoe group, within the belladonna group by Braby & Pierce (2007). 3 However, it had weak support and grouped loosely with D. sagessa Fruhstorfer, so is here given unique species group ranking. The eichhorni clade was the focus of a study by Morinaka et al. (2002), who demonstrated its monophyly, although only a single gene, ND5, was used in their analysis. We only sampled three species from this group (D. eichhorni Rothschild & Jordan, D. germana Roepke and D. gilliardi Sanford & Bennett), the grouping of which coincided with that of Morinaka et al. (2002). The sagessa clade, herein newly recognized, is another distinctive group of just a few species, none of which were sampled by Braby & Pierce (2007). Indeed, they retained the classification of earlier authors, who considered D. sagessa (and allies) to be within the geraldina group, although Talbot (1928–1937) suggested that D. sagessa was not well placed and might belong to the kummeri [nigrina] species group. The unique status given here is supported by the sagessa group possessing very distinctive genitalia, with long apical processes of the valva, quite different to members of the geraldina clade. In the present study, the geraldina clade is taken to include only a few members of the large, heterogeneous group of species originally proposed by Talbot (1928–1937) and adopted by Yagishita et al. (1993) but comprises all three members of the previously recognized stresemanni group. Indeed, Talbot (1928–1937) grouped D. stresemanni Rothschild and D. lecerfi Joicey & Talbot on genitalic and androconial affinities. Braby & Pierce (2007) grouped the cunningputi clade with (geraldina + stresemanni) but the geraldina clade recognized herein is supported as a clade distinct from the aroae (cunningputi) groups. The revised phylogeny shows that both this and the aroae group contain species that were assigned to the geraldina group by previous authors. The subgenus Piccarda Grote was restored for the hyparete clade by Talbot (1928–1937), based on characteristic androconial discs and male genitalia, and is a well supported group in the present study. The species D. eileenae Joicey & Talbot, which resembles some members of the dorimene group and was hence included in that group by Talbot (1928–1937), was rightfully transferred to the hyparete group by Yagishita et al. (1993). The belisama clade is another well-supported group but its relationships were poorly resolved in the analysis of Braby & Pierce (2007). In our analysis it is sister to (albertisi + nigrina). The albertisi clade comprises the niepelti, 4 iltis, clathrata, albertisi and bornemanni groups of previous authors. We found that there is much overlap of these groups, so their separation is not warranted. Shared morphological characters are also present between the species (e.g. those of D. albertisi Oberthür and D. discus Honrath with D. elongatus Kenrick). The albertisi and nigrina clades were broadly grouped by Braby & Pierce (2007). However, both are distinct, well-supported groups. Within the nigrina clade, both D. harpalyce Donovan and D. wollastoni Rothschild were recovered as outlying members, although the sequence (only COI) for the latter is very short. Talbot (1928–1937) also suggested D. wollastoni was an outlying member of the nigrina group, based on examination of the then unique type. Delias mayrhoferi Bang-Haas (=schunichii Morita) was considered of uncertain status (e.g. Gotts & Ginn, 2003) but in our analysis, it is sister to D. eximia Rothschild. Barcoding Delias Delias are characterized by several species groups containing numbers of species that are phenotypically very similar, i.e. cryptic species. This is particularly evident within the albertisi and nigrina species groups, all of which are endemic to the uplands of mainland New Guinea. We assessed three sets of sibling species, using the COI gene using two to four exemplars of each species. In all cases, we attempted to use specimens from two locations where the species occur in sympatry. The first set of species from the nigrina clade (D. kummeri Ribbe, D. ligata Jordan and D. isocharis Rothschild & Jordan), well known for their subtle but consistent differences of pattern on the underside hindwing, are widespread throughout much of the central cordillera of New Guinea. For both MP and Bayesian analyses (see Fig. S1a and S1b, respectively, below), specimens of each of these species grouped with high support and the genetic differences between them averaged 3.9%, 3.2% and 3.4% for kummeri-ligata, kummeri-isocharis and ligata-isocharis, respectively. Delias ligata was originally described as a form of D. kummeri but Talbot (1928–1937) noted differences in the androconia and male valva and that there were no intermediates. Delias weiskei Ribbe and D. leucias Jordan, also in the nigrina clade, are sister taxa exhibiting few external differences and have extensively overlapping distributional ranges in mainland New Guinea. Our analysis showed a genetic divergence averaging 5 3.3% between the taxa. A similar scenario was recorded for the group of closely related species in the albertisi clade, namely D. callista Jordan, D. iltis Ribbe, D. hapalina Jordan and D. luctuosa Jordan. Despite the similar external facies, the pairwise divergence was significant between these species, with averages of 6.0% (callista-luctuosa), 4.9% (callista-iltis), 4.0% (luctuosa-iltis), 7.1% (callista-hapalina), 4.0% (luctuosa-hapalina) and 3.3% (iltis-hapalina). The utility of the COI marker for barcoding cryptic species has been controversial. In the case of Delias, the COI gene shows high definition and resolves the relationships between closely related species. Müller et al. (2010) showed that it had variable application for delineation of closely related species of Charaxes (Nymphalidae) in the region but generally resolved relationships. REFERENCES Braby, M.F. & Pierce, N.E. (2007) Systematics, biogeography and diversification of the Indo-Australian genus Delias Hübner (Lepidoptera: Pieridae): phylogenetic evidence supports an ‘out-of-Australia’ origin. Systematic Entomology, 32, 2-25. D’Abrera, B. (1971) Butterflies of the Australian region. Lansdowne, Melbourne. D’Abrera, B.L. (1977) Butterflies of the Australian region, 2nd edn, Lansdowne, Melbourne. D’Abrera, B.L. (1990) Butterflies of the Australian region, 3rd (revised) edn. Hill House, Melbourne. Gotts, R.I. C. & Ginn, S.G. (2003) The previously undescribed female of Delias schunichii Morita (Lepidoptera: Pieridae) from New Britain, Papua New Guinea. Australian Entomologist, 30, 1-4. 6 Miller, L.D., Simon, M.J. & Wills, L. (2007) A new species of Delias (Lepidoptera: Pieridae) from New Ireland, Bismarck Islands, Papua New Guinea. Bulletin of the Allyn Museum, 144, 1-5. Morinaka, S., Miyata, T. & Tanaka, K. (2002) Molecular phylogeny of the eichhorni group of Delias Hübner, 1819 (Lepidoptera: Pieridae). Molecular Phylogenetics and Evolution, 23, 276-287. Morishita, K. (1981) The belladonna group of the genus Delias (Lep. Pieridae). Rhopalocerists’ Magazine, 4, 2-35. Müller, C.J. (2001) A new species of Delias Hübner (Lepidoptera: Pieridae) from New Ireland, Papua New Guinea. Australian Entomologist, 28, 17-22. Müller, C.J., Wahlberg, N. & Beheregaray, L.B. (2010) ‘After Africa’ – The evolutionary history and systematics of the genus Charaxes Ochsenheimer (Lepidoptera: Nymphalidae) in the Indo-Pacific Region. Biological Journal of the Linnean Society, 100, 457-481. Talbot, G. (1928–37) A monograph of the pierine genus Delias, Parts I–VI. British Museum (Natural History), London. Yagishita, A., Nakano, S. & Morita, S. (1993) An illustrated list of the genus Delias Hübner of the world. Khepera Publishers, Tokyo. Yata, O. (1985) Part 1: Pieridae. Butterflies of the South East Asian Islands. II: Pieridae, Danaidae (ed. by E. Tsukada), pp. 33–120, 205–438. Plapac, Tokyo. 7 Figure S3 Phylogenetic trees for the COI dataset of selected members of the nigrina and albertisi species groups. (a) Strict consensus tree of 53 equally parsimonious trees. Numbers below branches are bootstrap values > 50% for the node to the right. (b) Fifty percent majority rule consensus phylogram from the Bayesian analysis. Numbers to the left of nodes are the posterior probabilities of those nodes. (a) 8 (b)