Exam_6
advertisement
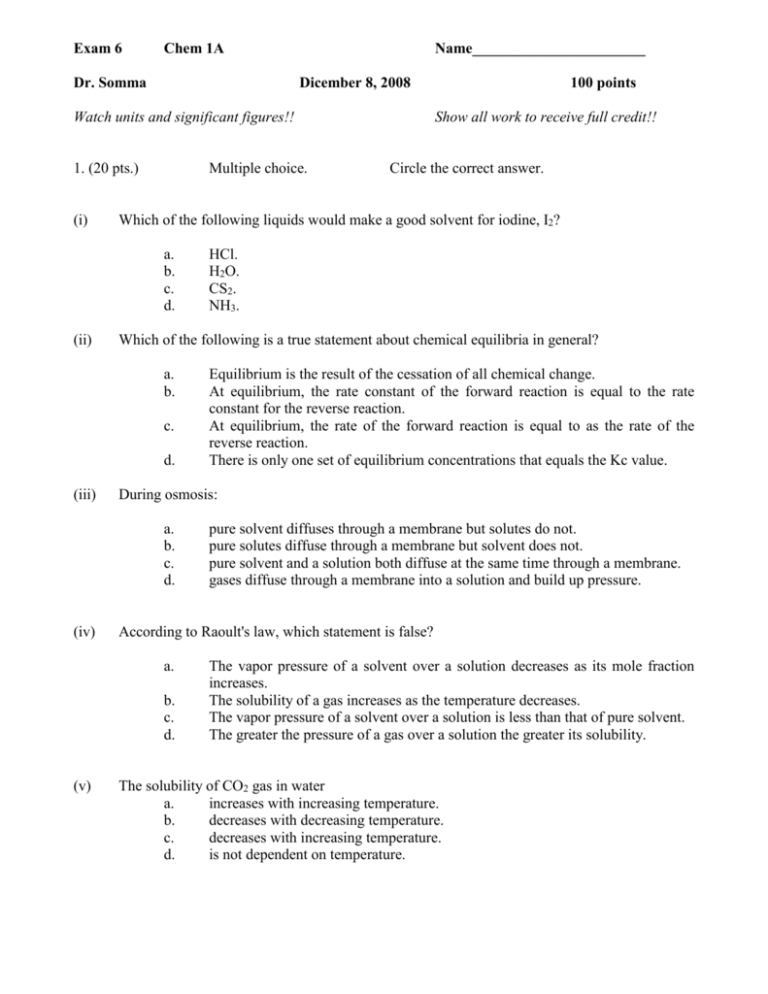
Exam 6 Chem 1A Dr. Somma Name_______________________ Dicember 8, 2008 Watch units and significant figures!! 1. (20 pts.) (i) Multiple choice. c. d. Equilibrium is the result of the cessation of all chemical change. At equilibrium, the rate constant of the forward reaction is equal to the rate constant for the reverse reaction. At equilibrium, the rate of the forward reaction is equal to as the rate of the reverse reaction. There is only one set of equilibrium concentrations that equals the Kc value. pure solvent diffuses through a membrane but solutes do not. pure solutes diffuse through a membrane but solvent does not. pure solvent and a solution both diffuse at the same time through a membrane. gases diffuse through a membrane into a solution and build up pressure. According to Raoult's law, which statement is false? a. b. c. d. (v) HCl. H2O. CS2. NH3. During osmosis: a. b. c. d. (iv) Circle the correct answer. Which of the following is a true statement about chemical equilibria in general? a. b. (iii) Show all work to receive full credit!! Which of the following liquids would make a good solvent for iodine, I2? a. b. c. d. (ii) 100 points The vapor pressure of a solvent over a solution decreases as its mole fraction increases. The solubility of a gas increases as the temperature decreases. The vapor pressure of a solvent over a solution is less than that of pure solvent. The greater the pressure of a gas over a solution the greater its solubility. The solubility of CO2 gas in water a. increases with increasing temperature. b. decreases with decreasing temperature. c. decreases with increasing temperature. d. is not dependent on temperature. 2. (12 pts.) Suppose the ions in a two-dimensional ionic crystal have the following arrangement: a. On the drawing above, sketch the unit cell of this crystal. b. How many ions are in this unit cell? c. What is the coordination number of each ion in this crystal? 3. (12 pts.) The density of an aqueous solution containing 10% of ethanol (C2H5OH) by mass is 0.984 g/mL a. Calculate the molality and the molarity of this solution. b. What volume of the solution would contain 0.125 mole of ethanol? (Hint: assume to work with 100 g of solution) 4. (14 pts) At 20 oC the vapor pressure of methanol (CH3OH) is 94 torr and the vapor pressure of ethanol (C2H5OH) is 44 torr. Being closely related these two compounds form a two components system which obeys to Raoult’s law throughout the entire range of concentrations. a. If 20 g of CH3OH is mixed with 100 g of C2H5OH determine the partial pressure exerted by each component and the total pressure of the solution. b. Calculate the composition of the vapor above the solution by applying the Dalton’s law. 5. (14 pts.) Consider the following decomposition reaction in a closed container: CaCO3 (s) CaO(s) +CO2 (g) Use the Le Chatelier principle to predict in which direction the equilibrium shifts in response to the indicated change in conditions: a. the volume is increased. ____________________________ b. a little amount of CaO is added to the mixture. ____________________________ c. all CaCO3 is removed. ____________________________ d. some CO2 is added to the mixture. ____________________________ e. the temperature is increased. ____________________________ f. some He is added at constant volume ____________________________ g. a few drops of a HCl solution are added to the mixture. ____________________________ 6. (16 pts.) The equilibrium constant Kc for the decomposition of phosgene, COCl2 is 4.63 x 10-3 at 527 oC. COCl2 (g) CO (g) + Cl2 (g) a. b. Calculate the corresponding equilibrium constant Kp. Use this information to calculate the equilibrium partial pressure of all components, starting with pure phosgene at 0.760 atm. 7. (12 pts.) When silver crystallizes, it forms face-centered cubic cells. The unit cell edge length is 408.7 pm. Calculate the density of silver