Genetics Lab – The Case of the Bad Burger
advertisement

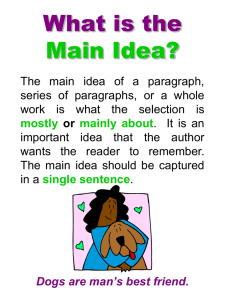
Module Mystery 3 – The Case of the Bad Burger The Case: Just when the poor folks in Virulent, Pennsylvania thought it was safe to go back to their daily lives, disease struck again! Approximately fifty people fell ill during a forty-eight hour period. Their symptoms included severe abdominal pain, nausea, cramping and vomiting. Health officials quickly ascertained that all fifty affected people had recently eaten at "Buff's Big Bad Burgers", where they had each consumed a double bad-boy burger. Buff loudly denied that his burgers could be to blame, and has agreed to let you test the burgers for the presence of E. coli DNA. Your Job: The Virulent Department of Health needs to know if the hamburger is indeed the source of the food poisoning, and, if so, which bacterial species is responsible. This information can then be used to determine the probable source of the contamination. The following is a list of bacteria that are considered likely “suspects”: Salmonella enterica (serotype typhimurium) Campylobacter jejuni Escherichia coli Listeria monocytogenes During the next three lab periods, you will 1) determine if the hamburger was contaminated using the polymerase chain reaction, 2) determine the type of bacterial contamination and, 3) support your conclusions using BLAST analysis of your gene sequences. Lab Report: The oral presentations for this module mystery will be during Week 14 (December 1-5). Procedure: Week One: Polymerase Chain Reaction In a 0.2 mL microcentrifuge tube, add: 25 L Ready Mix PCR Reaction Mix 2 L forward primer 2 L reverse primer 1 L hamburger DNA 20 L sterile water In a separate tube, add: Same as tube one, but 1 L of sterile water in place of the hamburger DNA (yes, this is your negative control). Place tubes in the thermal cycler and start FILE CK Conditions of file: 95°C for 4 min., then 94°C 1 min 55°C 1 min. Repeat 30 cycles 72°C 2 min then 72°C for 5 min. Week Two: Analysis of Products 1) Run a 1% agarose gel with EtBr in 1 X TAE to determine the size of your fragment(s). You will use the Hin dIII-digested Lambda DNA standard. 2) Load 5 uL of each sample, including the size standard, into each well and run at approximately 100 volts until the dye has migrated about half way down the gel. 3) Take a picture of your gel. Is the bacterial 16S rRNA gene fragment (around 798 bp) visible in any of your samples?