MHS Chemistry
advertisement

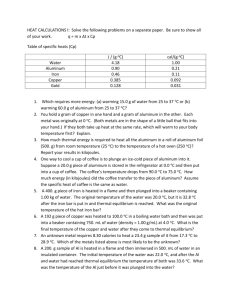
Stoichiometry Lab (Aluminum version) Name: _________________________ Aluminum metal reacts with hydrochloric acid to form hydrogen gas and an aluminum chloride solution. In this investigation, you will predict the mass of hydrogen gas produced by a given amount of reactants, then measure the amount, and compare them. Hydrochloric acid is an aqueous solution of hydrogen chloride. The concentration is usually described in terms of “moles per liter” which is abbreviated “mol/L” or “molar” or “M.” The most concentrated hydrochloric acid is 12 M, but for safety reasons we will be using a solution that is 3.0 M. Procedure 1. Put on a pair of safety goggles, and open your lab notebook to a new page. 2. Obtain approximately 0.25 grams of aluminum. (This will be a strip approximately 2cm long.) Record the exact mass, and cut it into tiny pieces. 3. Put 50.0 mL of the hydrochloric acid into a small beaker. Record the mass of the beaker with solution in it. 4. Place the foil pieces on the balance tray next to the beaker of acid, and record the total mass. [If this total mass does not equal the sum of number 2 and 3, you should remeasure.] 5. With the beaker still on the balance, drop the foil into the acid and observe. What happens to the overall mass? 6. When the aluminum is gone, record the final mass of the beaker with the solution. 7. Dispose of the materials in the beaker as follows: a. Turn on the faucet at the sink and pour excess liquid down the drain. Run the faucet to dilute the remaining acid. Be careful not to allow any remaining Al to fall in the sink. b. Partially fill the beaker with water, then pour it out in the sink, again being careful not to allow any aluminum to get in the sink/drain. c. Dispose of any remaining aluminum in the trash can. Analysis 1. Write the chemical equation for the reaction that took place. Balance it. 2. Calculate the number of moles of (clean) aluminum used. 3. Predict the number of moles of hydrogen that should be produced by this much aluminum reacting. 4. Calculate the mass of hydrogen gas produced during your reaction. 5. Calculate the number of moles of hydrogen gas actually produced. 6. Calculate the “percent yield” for your reaction. If your prediction (#3) and calculation (#5) were the same, your percent yield was 100%. 7. Why was your percent yield what it was? In other words, if you got 100%, why was it so perfect? If you got less than 100%, what happened?