Protocol for Culture of Murine ES Cells – Leeat Anker
advertisement
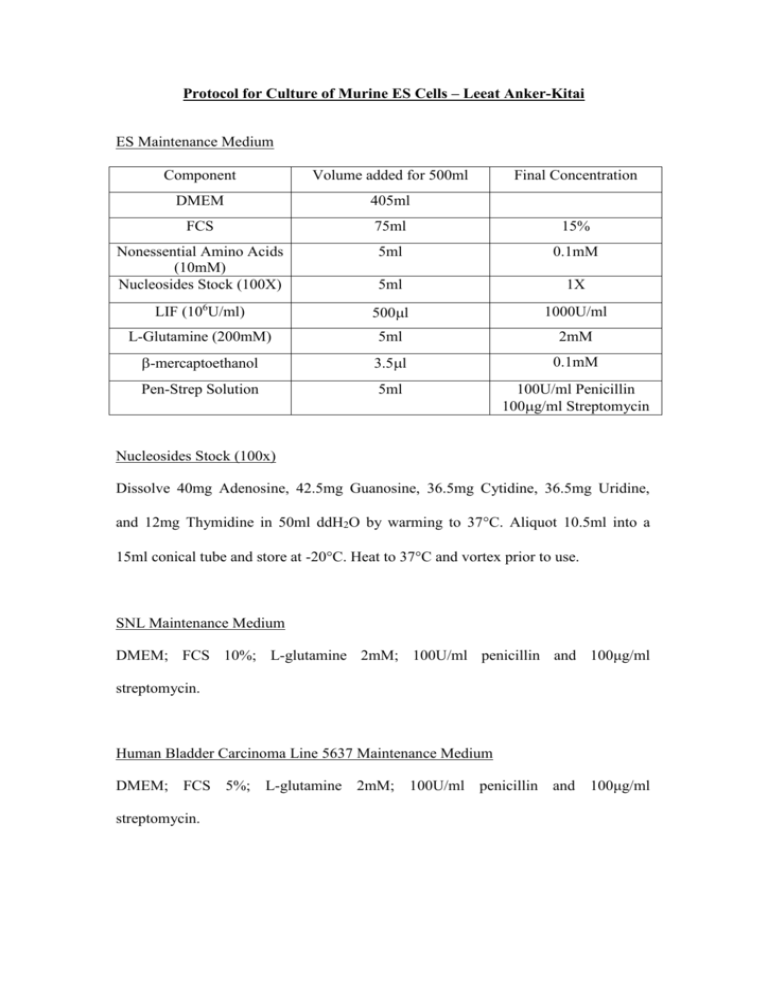
Protocol for Culture of Murine ES Cells – Leeat Anker-Kitai ES Maintenance Medium Component Volume added for 500ml Final Concentration DMEM 405ml FCS 75ml 15% Nonessential Amino Acids (10mM) Nucleosides Stock (100X) 5ml 0.1mM 5ml 1X LIF (106U/ml) 500l 1000U/ml L-Glutamine (200mM) 5ml 2mM -mercaptoethanol 3.5l 0.1mM Pen-Strep Solution 5ml 100U/ml Penicillin 100g/ml Streptomycin Nucleosides Stock (100x) Dissolve 40mg Adenosine, 42.5mg Guanosine, 36.5mg Cytidine, 36.5mg Uridine, and 12mg Thymidine in 50ml ddH2O by warming to 37C. Aliquot 10.5ml into a 15ml conical tube and store at -20C. Heat to 37C and vortex prior to use. SNL Maintenance Medium DMEM; FCS 10%; L-glutamine 2mM; 100U/ml penicillin and 100μg/ml streptomycin. Human Bladder Carcinoma Line 5637 Maintenance Medium DMEM; FCS 5%; L-glutamine 2mM; 100U/ml penicillin and 100μg/ml streptomycin. Mitomycin C (1mg/ml) Dissolve 2mg Mitomycin C in 2ml PBS. Prepare 100μl aliquots (100X) and store at -20ºC. Gelatin Solution 0.1% (w/v) Dissolve 300mg gelatin in 300ml ddH20. Sterilize by autoclaving. Preparation of LIF-Containing Conditioned Medium for ES Cells Background: LIF is the cytokine that ES cells require in order to remain as an undifferentiated proliferating population of cells. An alternative source of the commercially available recombinant protein form (ESGRO) is from cells that synthesize and secrete LIF into the culture medium. Such conditioned medium can be concentrated or used directly (at the appropriate dilution) as a supplement to the feeder cells or as a substitute. Human bladder carcinoma line 5637 can be obtained from the American Type Culture Collection (ATCC, Rockville, MD). Protocol: 1. The 5637 cells are grown in standard tissue culture flasks/dishes at 37°C in DMEM plus 5% FCS. 2. Grow the cells to confluency. Large cultures may be grown in roller bottles or cell factories, T-75 flasks, or 175 flasks depending on the volume required. 3. Once the cells have reached confluency, fresh medium is added, and the cells are cultured for 3-4 days before the medium is collected. Fresh medium is immediately added to the cells, and repeated collections can be made twice a week for up to 6 weeks. 4. The collected medium is centrifuged at 12,000-16,000 rpm for 1 hour to remove cells and any insoluble material. 5. Filter through a 0.45-μm sterile filter and store at - 20°C until required. 6. 100 ml of conditioned medium for growing ES cells is made up as follows: - 65 ml of sterile-filtered 5637-conditioned medium - 23 ml of DMEM - 12 ml of FCS - MEM non-essential amino acids to final concentration of 0.1 mM [from 100x (10 mM) stock solution, GIBCO-BRL] - Penicillin/streptomycin (100x liquid, GIBCO-BRL) - L-Glutamine - 2-Mercaptoethanol at 0.1 mM from 0.1 M stock (200 mM from 100x stock, GIBCO-BRL) Inactivation of SNL Feeder Layer with Mitomycin C: Background: Mitomycin C cross-links DNA and blocks cell proliferation. It a potent carcinogen and mustn’t be inhaled or handled. Mitomycin C stock (0.5-1.0mg/ml in PBS or glass distilled water) is used. It can be stored for 1 week at 4C protected from light (i.e. wrapped in foil). If stored frozen, check that it is all in solution after thawing. Protocol: 1. Treat confluent SNL cells in tissue culture dishes with freshly prepared DMEM plus 10% FCS (fetal calf serum) and 10g/mL of mitomycin C 2. Return the dishes to the humidified incubator at 37C with 5% CO2 for 2-3 hours 3. In the meantime prepare tissue culture dishes with gelatin. This is done by flooding with a 0.1% solution of gelatin for 2 hours. Remove the gelatin and allow the plates to dry. This increases the adhesiveness of the feeder cells to the dish 4. Wash the dishes extensively with several changes (x 3) of PBS and collect the cells by trypsinization 5. Pellet the cells by low-speed centrifugation (1000 rpm for 5 minutes), resuspend the pellet with fresh DMEM plus 10% FCS 6. Count the cells and dilute them to give a final cell density of 3 x 105/mL* 7. Plate the cells onto tissue culture dishes pre-treated with gelatin * Note: It is important to place the fibroblasts at the correct density to ensure that a confluent uniform monolayer is produced. A density of 5 x 104/cm2 is suggested as being suitable for STO cells (or SNL cells). An approximate guide: 6-cm plates - 1.0 x 106 cells; 10-cm plates – 3.0 x 106 cells. Feeder plates may be used for up to 10 days after they are made, and the medium should be replaced immediately before use with the ES cell culture medium. Check for the integrity of the monolayer before use. Differentiation Protocol for ES Cells ES Differentiation Medium 1 (with Serum) As for ES Maintenance Medium, without LIF ES Differentiation Medium 2 (without Serum) As for ES Maintenance Medium, without LIF, without serum, with 15% Serum Replacement (SR). Tissue Culture Media and Solutions Adenosine, Cytidine, Guanosine, Thymidine, Uridine, Sigma Chemical Co. St Louis, MO Dulbecco’s Modified Eagle’s Medium (DMEM) with Glucose 4500mg/l, w/o Sodium Pyruvate, w/o L-Glutamine, Gibco Invitrogen Corporation, Grand Island, NY Dulbecco’s Phosphate Buffered Saline (PBS), Biological Industries, Beit Haemek, Israel Fetal Calf Serum (FCS) Heat-Inactivated, Biological Industries, Beit Haemek, Israel Gentamycin Sulphate 50mg/ml, Biological Industries, Beit Haemek, Israel KNOCKOUT™ Serum Replacement (SR), Gibco Invitrogen Corporation, Grand Island, NY L-Glutamine Solution 200mM, Biological Industries, Beit Haemek, Israel Leukemia Inhibitory Factor (LIF ESGROTM) 107 units, Chemicon International, Temecula, CA MEM (100X) Non-Essential Amino Acids, Gibco Invitrogen Incorporation, Grand Island, NY 2-Mercaptoethanol (β-Mercaptoethanol) 14.3M, Sigma Chemical Co. St Louis, MO PBS (Mg and Ca free), Biological Industries, Beit Haemek, Israel Pen-Strep Solution (Penicillin: 10,000 units/ml; streptomycin: 10mg/ml), Biological Industries, Beit Haemek, Israel Trypsin-EDTA (0.25% Trypsin 1mM EDTA), Gibco Invitrogen Corporation, Grand Island, NY Versene-Trypsin Solution, Bio Lab Ltd., Jerusalem, Israel Pre-Differentiation 48 hours prior to the onset of differentiation, 2 x 106 ES cells (transfected and non-transfected) were counted and passaged onto 100-mm gelatinized dishes in ES Maintenance Medium and grown for two days. Embryoid Body Formation In order to allow the formation of embryoid bodies (EBs), stably-transfected and non-transfected ES cells were transferred to Petri dishes (2 dishes for each) and were grown in suspension for 6 days in ES Differentiation Medium 1 (with serum). Re-attachment of EBs and Serum Replacement Medium In the next step, EBs were transferred to 100-mm tissue culture dishes in ES Differentiation Medium 2 (with SR) to enhance cell differentiation. This was done by transferring the EBs from Petri dishes to 15-ml tubes, where they were left to stand for 3-4 minutes to allow sedimentation at the bottom of the tube. Medium was then removed and EBs from each Petri dish were replated on regular 100-mm tissue culture dishes for the remainder of the experiment (11-38 days).








