alkenes

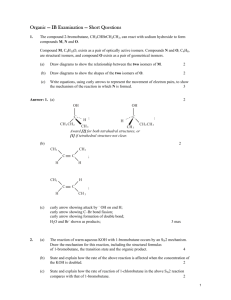
Structural Isomers
“Iso” – the same
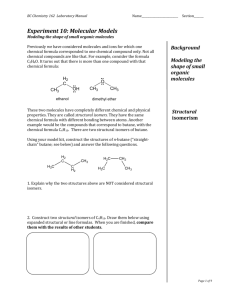
Structural isomers are compounds that have the same molecular formula but different molecular structures.
Ex: Isomers for C
4
H
10
Ex: Isomers for C
5
H
12
Now you try: Draw all structural isomers for C
6
H
14
. Name each one.
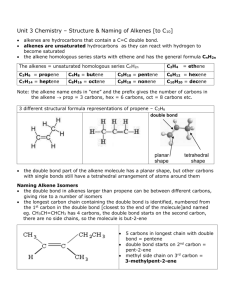
ALKENES
Alkenes are organic compounds that contain double bonds between carbon atoms
The general formula for an alkene is C n
H
2n
.
Unsaturated compound: an organic compound that contains double or triple C-C bonds
(contains less than the maximum possible # of H’s in its structure)
Saturated compound: an organic compound that contains the maximum # of H’s, therefore having all single C-C bonds.
Naming Alkenes:
1. Find the longest continuous chain containing the double bond. This is the parent alkene. It gets the root name of the alkane with the same number of carbons but with the ending ‘-ene’.
2. For chains with more than 3 carbons, number the carbons so that the carbons with the double bond get the lowest number.
3. Substituents on the chain are numbered and named in the same way as for alkanes.
Ex:
e)
Try: Name the following. a) c) b) d)
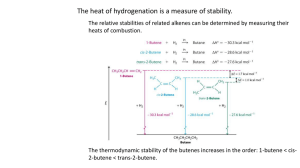
Geometric Isomers
Geometric isomers are compounds that have the same molecular formula but differ in the geometry (orientation in 3D space) of their substituted groups
in ethane, the C-C bond can rotate
geometric isomers not possible
in ethene, the C=C bond can not rotate, so geometric isomers can form; the location of substituents in relation to the double bond can differ!
Trans configuration – the substituted groups are on opposite sides of the double bond
Cis configuration – the substituted groups are on the same side of the double bond
Example: 2-butene
** If you are drawing structural formulas for alkenes and cis/trans forms exist, you must draw both.
Ex1: Draw 2-pentene
Ex 2: Draw 2-methyl-1-butene
7.
5.
3.
Name the following alkenes:
1.
Alkenes Practice Problems
2.
4.
6.
8.
2. Draw structural formulas for the following alkenes. If a compound has geometric isomers, draw both the cis and trans forms.
1. 1-pentene
2. 2-hexene
3. 2-methyl-2-hexene
4. 2,3-dimethyl-2-butene
5. 5,6-dimthyl-2-octene