Experiment1:
advertisement
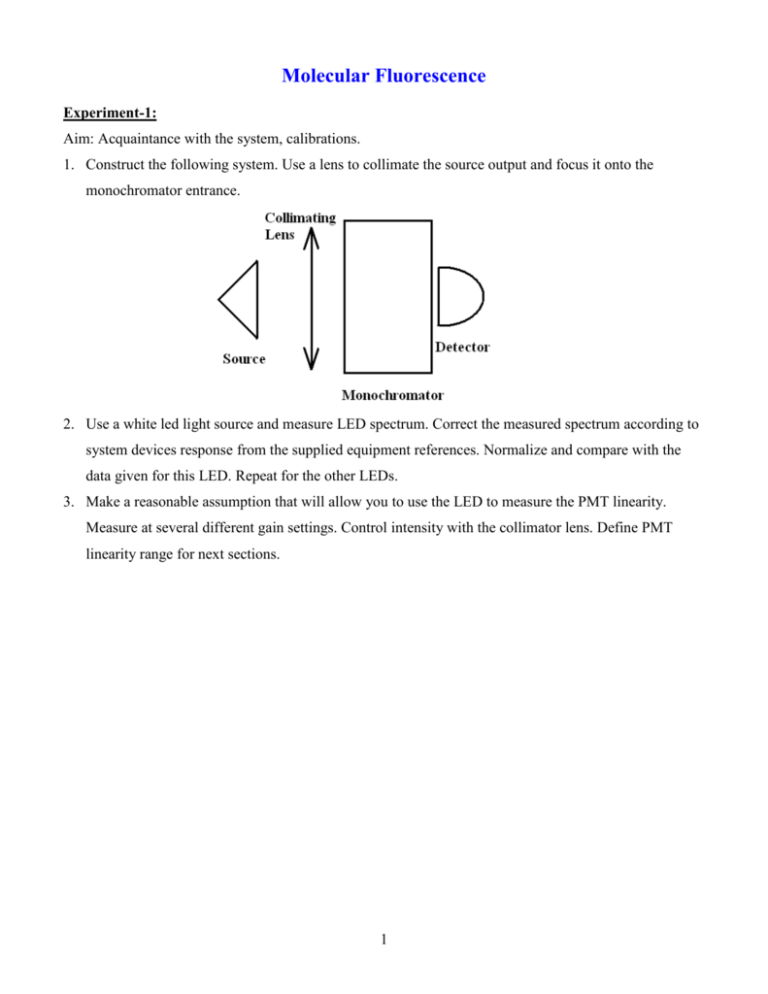
Molecular Fluorescence Experiment-1: Aim: Acquaintance with the system, calibrations. 1. Construct the following system. Use a lens to collimate the source output and focus it onto the monochromator entrance. 2. Use a white led light source and measure LED spectrum. Correct the measured spectrum according to system devices response from the supplied equipment references. Normalize and compare with the data given for this LED. Repeat for the other LEDs. 3. Make a reasonable assumption that will allow you to use the LED to measure the PMT linearity. Measure at several different gain settings. Control intensity with the collimator lens. Define PMT linearity range for next sections. 1 Experiment-2: Aim: Beer-Lambert absorption and Fluorescence. 1. Construct the following system: The “?” near “Filter” indicate a non-compulsory component. There are several vials with different concentrations of fluorescein in distilled water (pH 7.0), and one with an unknown concentration. 2. Obtain the Beer-Lambert extinction-coefficient (=514nm) for fluorescein in water. 3. Measure and calculate the concentration of the fluorescein in the vial marked “?mM”. 4. Look at the light that goes through the sample, at different concentrations. What is happening? 2 Experiment-3 Obtain the emission spectra of fluorescein. The aim of this experiment is to acquaint you with the various difficulties of measuring fluorescence. 1. Construct the system in the figure: 2. Measure the emission spectrum of Fluorescein. Use the vial which says 0.01mM. The maximal intensity is at 514nm, you may use high gain but Watch it! Gain 10 is the highest PMT gain. If exposed to too much light, the PMT could be damaged! Normalize your result and put the tabulated fluorescein spectrum (you find it in the webpage) on top of your measured spectrum. Explain all the differences between the two in your report in terms of how did the extra-light get to some wavelengths and why is it lacking in the other wavelengths. 3. It is a HUGE hint, to put the detector at 90 to the excitation beam. What would happen if we placed the source, detector and sample on a single line? 4. Repeat with a higher concentration of fluorescein (the vial that says ?mM and by now you know its concentration). Explain the difference between the data from webpage, your previous measurement and this one. 5. What would happen if we excited with a different wavelength? 3 Experiment-4 Aim: find the depolarization angle and the viscosity 1. Construct the following system: 2. Obtain the polarization of fluorescein in glycerin at various concentrations: 0%-80% and in water. Explain the results. 3. Find the viscosities of the solutions at each concentration and compare to data 4













![This article was downloaded by: [Oregon State University]](http://s2.studylib.net/store/data/010881326_1-423cbd75b0b228bbc400ac8651cb3851-300x300.png)