IRB PROTOCOL REVIEW STANDARDS.revised 2.1.13 (2)
advertisement
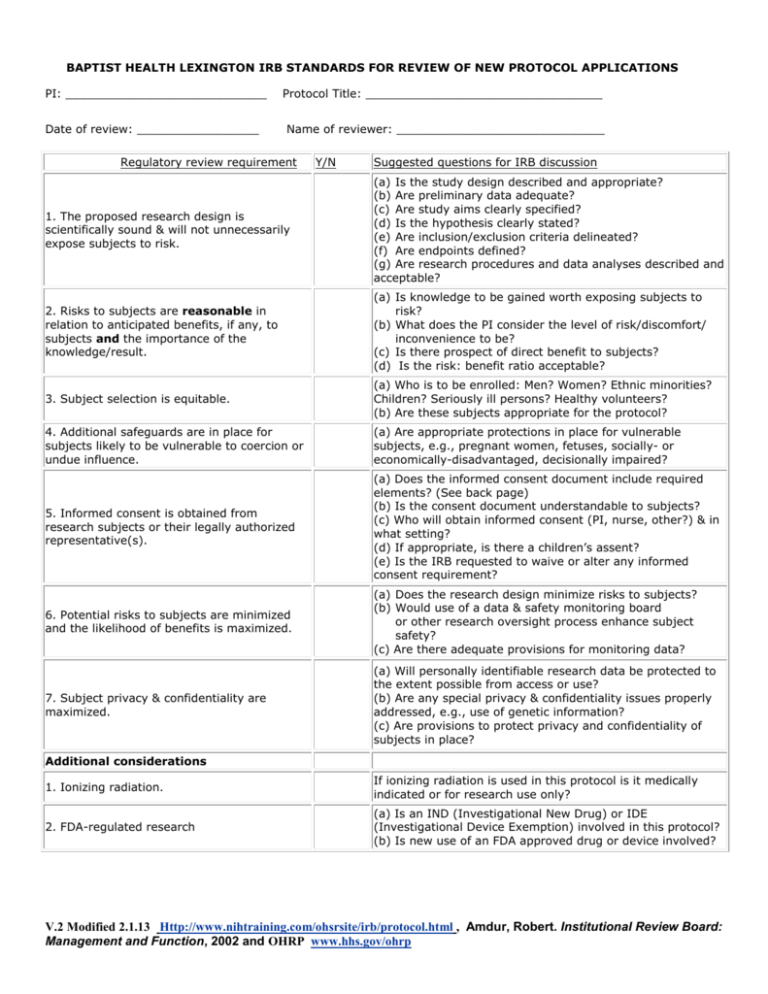
BAPTIST HEALTH LEXINGTON IRB STANDARDS FOR REVIEW OF NEW PROTOCOL APPLICATIONS PI: ____________________________ Date of review: _________________ Protocol Title: _________________________________ Name of reviewer: _____________________________ Regulatory review requirement Y/N Suggested questions for IRB discussion 1. The proposed research design is scientifically sound & will not unnecessarily expose subjects to risk. (a) Is the study design described and appropriate? (b) Are preliminary data adequate? (c) Are study aims clearly specified? (d) Is the hypothesis clearly stated? (e) Are inclusion/exclusion criteria delineated? (f) Are endpoints defined? (g) Are research procedures and data analyses described and acceptable? 2. Risks to subjects are reasonable in relation to anticipated benefits, if any, to subjects and the importance of the knowledge/result. (a) Is knowledge to be gained worth exposing subjects to risk? (b) What does the PI consider the level of risk/discomfort/ inconvenience to be? (c) Is there prospect of direct benefit to subjects? (d) Is the risk: benefit ratio acceptable? 3. Subject selection is equitable. (a) Who is to be enrolled: Men? Women? Ethnic minorities? Children? Seriously ill persons? Healthy volunteers? (b) Are these subjects appropriate for the protocol? 4. Additional safeguards are in place for subjects likely to be vulnerable to coercion or undue influence. (a) Are appropriate protections in place for vulnerable subjects, e.g., pregnant women, fetuses, socially- or economically-disadvantaged, decisionally impaired? 5. Informed consent is obtained from research subjects or their legally authorized representative(s). (a) Does the informed consent document include required elements? (See back page) (b) Is the consent document understandable to subjects? (c) Who will obtain informed consent (PI, nurse, other?) & in what setting? (d) If appropriate, is there a children’s assent? (e) Is the IRB requested to waive or alter any informed consent requirement? 6. Potential risks to subjects are minimized and the likelihood of benefits is maximized. (a) Does the research design minimize risks to subjects? (b) Would use of a data & safety monitoring board or other research oversight process enhance subject safety? (c) Are there adequate provisions for monitoring data? 7. Subject privacy & confidentiality are maximized. (a) Will personally identifiable research data be protected to the extent possible from access or use? (b) Are any special privacy & confidentiality issues properly addressed, e.g., use of genetic information? (c) Are provisions to protect privacy and confidentiality of subjects in place? Additional considerations 1. Ionizing radiation. If ionizing radiation is used in this protocol is it medically indicated or for research use only? 2. FDA-regulated research (a) Is an IND (Investigational New Drug) or IDE (Investigational Device Exemption) involved in this protocol? (b) Is new use of an FDA approved drug or device involved? V.2 Modified 2.1.13 Http://www.nihtraining.com/ohsrsite/irb/protocol.html , Amdur, Robert. Institutional Review Board: Management and Function, 2002 and OHRP www.hhs.gov/ohrp RISK:BENEFIT ASSESSMENT RISK refers to the probability of harm or injury (physical, psychological, social or economic) that could occur as a result of participation in a research study. Both the probability and magnitude of possible harm may vary from minimal to significant. Federal regulations define only "minimal risk." Minimal risk means that the probability and magnitude of harm or discomfort anticipated in the research are not greater in and of themselves than those ordinarily encountered in daily life or during the performance of routine physical or psychological examinations or tests (45 CFR 46.102(h)(i)). Research methodologies and procedures are distinguished from risks associated with therapies the subject would undergo even if not participating in research. A research BENEFIT is considered to be something of health-related, psychosocial or other value to an individual, or something that will contribute to society as a whole through increased knowledge, improved safety, technological advances and/or better health. Indicate which of the categories listed below accurately describes this study protocol: ____ Not greater than minimal risk ____ Greater than minimal risk, but presenting the prospect of direct benefit to individual subjects ____ Greater than minimal risk, no prospect of direct benefit to individual subjects, but likely to yield generalizable knowledge about the subject’s disorder or condition Eight Elements Mandatory in Every Protocol Consent Document per 45 CFR 46.116 Check and initial appropriate answer: Y__ N__ 1. A statement that participation is voluntary, that refusal to participate will involve no penalty or loss of benefits to which the subject is otherwise entitled, and that the subject may discontinue participation at any time without penalty or loss of benefits to which the subject is otherwise entitled. Y__ N__ 2. A statement describing the extent, if any, to which confidentiality of records identifying the subject will be maintained. Y__ N__ 3. For research involving more than minimal risk, an explanation of whether any compensation and any medical treatments are available if an injury occurs and, if so, what they consist of, or where further information may be obtained. Y__ N__ 4. An explanation of whom to contact for answers to pertinent questions about the research and research subjects’ rights, and who to contact if a subject suffers a research-related injury. Y__ N__ 5. A statement that the study involves research, an explanation of the purposes for the research, the expected duration of the subject’s participation, a description of the procedures to be followed, and identification of any procedures that is experimental. Y__ N__ 6. A description of any reasonably foreseeable risks or discomforts to the subject. Y__ N__ 7. A description of any benefits to the subject or to others that may reasonably be expected from the research. Y__ N__ 8. A disclosure of appropriate alternative procedures or courses of treatment, if any, that might be advantageous to the subject. V.2 Modified 2.1.13 Http://www.nihtraining.com/ohsrsite/irb/protocol.html , Amdur, Robert. Institutional Review Board: Management and Function, 2002 and OHRP www.hhs.gov/ohrp