The study of the negative thermal expansion of cuprite
advertisement

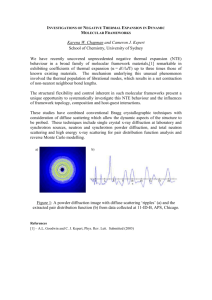
Study of the negative thermal expansion of cuprite-type structures by means of temperaturedependent Pair Distribution Function analysis: preliminary results Monica Dapiaggi1, HyunJeong Kim2, Emil S. Božin2, Simon J.L. Billinge2, Asel Sartbaeva3, Stephen A. Wells3, Gilberto Artioli4 1 Università degli Studi di Milano, Dipartimento di Scienze della Terra, Italy 2 Michigan State University, Department of Physics and Astronomy, USA 3 Arizona State University, Center for Biological Physics, USA 4 Università degli Studi di Padova, Dipartimento di Geoscienze, Italy The cuprite structure is a fairly simple structure shared by cuprite (Cu2O) and silver(I) oxide (Ag2O) and it can be described by two interpenetrated chains of corner sharing M4O tetrahedra, where M represents the metal atom. Both compounds show a wide range of negative thermal expansion (NTE): cuprite contracts with temperature up to about 200 K and then expands, while Ag2O has a NTE up to its decomposition temperature, at about 450 K. These materials have been investigated extensively by coupling the atomic displacement parameters measured by diffraction to an accurate EXAFS analysis of higher order cumulants in the whole accessible temperature range (for the latest results see [1] and [2]). Both structures show a complex local behavior of the metal atoms, with a positive thermal expansion of M-O bonds, coupled with a negative thermal expansion of only 6 of the 12 second nearest neighbors (M-M). Each tetrahedral unit proved to be, through the comparison with the thermal vibrations evaluated by diffraction, much stiffer against stretching than against bending. The strong anisotropy in the thermal vibrations of bridging M atoms confirmed to be related to NTE, but a more complex model is necessary to explain NTE in these materials. In the present work, temperature dependent PDFs of both compounds are compared and analyzed. The data were collected at the GEM beamline at ISIS (Rutherford Appleton Laboratory, Chilton, UK), with a liquid helium cryostat, from 5 K to about 400 K [3]. The PDF analysis yields structural information on various length-scales, and is sensitive to the presence of local structural distortions even when these are not observed crystallographically. The thermal evolution of the local and average structure can be of aid in understanding the complex thermal behavior of these materials and of many of negative thermal expansion materials. PDFGui and Pdffit2 [4] were used to refine the PDF data, together with a novel geometric refinement method [5]. This modeling approach uses simulated annealing to refine a model structure against the PDF data taking into account the local bonding geometry as a constraint. It allows large models to be compared to the data whilst controlling the number of independent degrees of freedom in a chemically reasonable way. The geometric modeling just described yields the following result, with a model structure based on units of 10x10x10 cells (about 50 Å wide): distorted regions of about 8-10 Å are included in a matrix having a rather ordered structure. The PDF fit in real-space gives a similar outcome, showing significant differences in the fit of the crystallographic model in the region below 10 Å. This is true for both the compounds, even though the temperatures at which the distortion starts to appear are very different: in Cu2O the first sign of distortion appears only at fairly high temperature (about 200 K), around the inversion region in thermal expansion, while for Ag2O the distortion is present even at the lowest temperature measured (5 K). The Ag2O sample measured showed no sign of the low-temperature phase transition observed in other samples [6, 7] References: 1. 2. 3. Sanson, A., Rocca, F., Dalba, G., Fornasini, P., Grisenti, R., Dapiaggi, M. & Artioli, G., 2006, Physical Review B: Condensed Matter and Materials Physics, 73, 214305/1-214305/13. Artioli, G., Dapiaggi, M., Fornasini, P., Sanson, A., Rocca, F. & Merli, M., 2006, Journal of Physics and Chemistry of Solids, 67, 1918-1922. Hannon, A.C., 2005, Nuclear Instruments & Methods in Physics Research, Section A: Accelerators, Spectrometers, Detectors, and Associated Equipment, 551, 88-107. 4. 5. 6. 7. Farrow, C.L., Juhas, P., Liu, J.W., Bryndin, D., Bloch, J., Proffen, T. & Billinge, S.J.L., 2006, Journal of Physics: Condensed Matter, submitted. Sartbaeva, A., Wells, S. A., Božin, E. S., Billinge, S. J. L. and Thorpe, M.F. unpublished Tiano, W., 2002, Laurea Thesis. Kennedy, B.J., Kubota, Y. & Kato, K., 2005, Solid State Communications, 136, 177-180.