POLA_24558_sm_suppinfo
advertisement

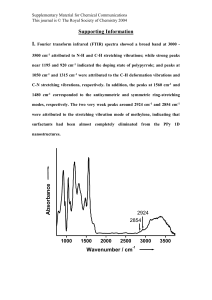
Supplementary Information Synthesis and Characterization of ABA-type Amphiphilic Triblock Copolymers through Anionic Polymerization using End Functionalized Poly(ethylene oxide) Oligomers PALASH JYOTI DAS1, ANIL BARAK1, YUSUKE KAWAKAMI2, AND THARANIKKARASU KANNAN3,* 1Department 2Graduate of Chemistry, North Campus, University of Delhi, Delhi-110 007, India. School of Materials Science, Japan Advanced Institute of Science and Technology, Asahidai 1-1, Tatsunokuchi, Ishikawa 923 1292, Japan. 3Department of Chemistry, Pondicherry University, R.V. Nagar, Kalapet, Puducherry – 605 014, India. Contents: 1) Synthesis of 1: 2) Synthesis of 1-(4-bromophenyl)-1-phenylethylene: 3) FT-IR spectra of (a) PEG, (b) 1, (c) SES 2 4) FT-IR spectra of (a) 2, (b) POLY 3, (c) PME 2 1 Synthesis of 1. As given in the Scheme S-1, 1 was synthesized from PEG in the presence of phosphorous tribromide as reported in the literature. The modified procedure to prepare 1 from PEG of molecular weight 1000 is presented here as reference procedure. PEG (16.0 g, 16.0 mmol) was taken in a 250 mL two necked round bottom flask in 500 mL of toluene. About 100 mL toluene was separated to remove the water. Then Phosphorous tribromide (PBr3) (1.5 mL, 16 mmol) was added over a period of 30 min and the mixture was refluxed for 8 h. The solution was concentrated by evaporation. The unreacted PBr3 was removed by applying vacuum of 10-6 torr at 60 °C. Then it was dissolve in diethylether and 1 was recrystalized from it. HO PBr3 O O n OH Br O O Toluene, 110°C n Br Scheme S-1. Synthesis of 1. Synthesis of 1-(4-bromophenyl)-1-phenylethylene. The synthesis of 1-(4-bromophenyl)-1phenylethylene was carried out as reported by Hairo et. al. The simplified procedure is as follows. The mixture of methyltriphenylphosphinebromide (18.85 g, 52 mmol) and tert-BuOK (7.7 g, 685 mmol) was taken in a two necked round bottom flask attached with separating funnel and 70 mL dry THF was added to it. p-Bromobenzophenone (12.5 g, 47.9 mmol) in 20 mL dry THF was taken in a separating funnel and it was added dropwise over a period of 30 min to the mixture of methyltriphenylphosphinebromide and tert-BuOK at 0 °C. Then the reaction mixture was stirred for 1 h at 0 °C followed by stirring for 2 h at room temperature. At the end of the reaction, the reaction was quenched by adding methanol. The crude product was purified by column chromatography on silica gel using hexane as a solvent and pure 1-(4-bromophenyl)-1-phenylethylene (DPE-Br) was obtained as a colourless liquid. 2 FT-IR spectra of PEG, 1, PS-b-PEO-b-PS tri-block copolymer, SES 3. In the FT-IR spectrum of 1 (cf. Figure S-1 (b)), the -C-H and C-O-C stretching vibrations present in CH2-CH2-O repeating units of PEO observed at 2853-3058 cm-1 and 1110 cm-1 respectively. To confirm the structure of the tri-block copolymer, SES3 was characterized by FT-IR spectroscopy and the spectrum is presented in Figure S-1 (c). Asymmetric and symmetric stretching vibration of -CH2 groups present in PS and PEO were observed at 2853-3058 cm-1. The stretching vibrations of -C-O-C- group present in the CH2-CH2-O repeating units of PEO block were observed at 1110 cm-1. In addition to these peaks, new peaks were observed at 3068-3092 cm-1, 1601 cm-1, 1454-1492 cm-1, and 757-698 cm-1 which are due to the -C-H stretching of phenyl ring, -C=C- stretching of phenyl ring, phenyl ring stretching and –C=C-H out of plane bending of phenyl ring present in the PS respectively. These new peaks are not present in the FTIR spectrum of 1. These results, again, confirm the formation of PS-b-PEO-b-PS tri-block copolymers. Figure S-1. FT-IR spectra of (a) PEG, (b) 1, (c) SES2. 3 FT-IR spectra of 2, PS-b-PEO-b-PS and PMMA-b-PEO-b-PMMA triblock copolymers. The formation of 2 was also confirmed by FT-IR spectroscopy. Figure S-2 shows the FT-IR spectra of 2, POLY 3 and PME 3. The -C-H asymmetric and symmetric stretching vibrations of -CH2 groups present in DPE and PEO block of DPE-PEO-DPE (2) were observed at 2853-3058 cm-1. The band at 1110 cm-1 is due to the stretching vibrations of -C-O-C- of PEO blocks. The new peaks observed at 3060-3090 cm-1, 1601 cm-1, 1454-1492cm-1, and 757-698cm-1 are due to the phenyl -C=C-H, phenyl -C=C-, phenyl ring stretching vibrations and phenyl -C=C-H out of plane bending vibrations of DPE group present in 2 respectively. The peak at 1642 cm-1 is due to the -C=C- stretching vibrations of vinyl group present in DPE. The presence of all the peaks to PEO and DPE moieties present in 2 further confirm the formation of 2. Figure S-2. FT-IR spectra of (a) 2, (b) POLY 3, (c) PME2. The FT-IR spectrum of the PS-b-PEO-b-PS tri-block copolymer, POLY 3 is presented in Figure S-2 (b). Asymmetric and symmetric -C-H stretching vibrations of -CH2 groups present in PS and PEO blocks are observed at 2853-3058 cm-1. The band at 1110 cm-1 is due to the stretching vibrations of -CO-C- of CH2-CH2-O repeating units of PEO block. The peaks at 3060-3090 cm-1, 1604 cm-1and 14541492 cm-1 are due to the -C-H stretching of phenyl ring, -C=C- stretching of phenyl ring and stretching of phenyl ring respectively. The -C=C-H out of plane bending of phenyl ring of PS block is observed at 757-698 cm-1. The peak at 1642 cm-1 for vinyl stretching of -C=C- of 2 is absent in the FT-IR spectrum 4 of PS-b-PEO-b-PS triblock copolymer which shows that polymerization of styrene was initiated through the ethylene group present in 2. The presence of the peaks for PEO and PS blocks in FT-IR spectrum confirm the formation of PS-b-PEO-b-PS triblock copolymers. Similarly PMMA-b-PEO-b-PMMA triblock copolymers were also characterized by FT-IR spectroscopy. In the FT-IR spectrum of PMMA-b-PEO-b-PMMA triblock copolymer, PME 3, (c.f Table 4), the asymmetric and symmetric -C-H stretching vibrations of -CH2 groups present in PEO and PMMA blocks are observed at 2853-3058 cm-1. The band at 1110 cm-1 is due to the stretching vibration of -C-O-C- of -CH2-CH2-O repeating units of PEO block. The peak at 1642 cm-1 which is due to the C=C- stretching vibrations of 2, is missing in the FT-IR spectrum of PMMA-b-PEO-b-PMMA triblock copolymer. A new peak appeared at 1734 cm-1, is due to the carbonyl group of PMMA block. The FT-IR spectra results further confirm the formation of PMMA-b-PEO-b-PMMA triblock copolymers. 5