Lesson 31 notes - Solids, Liquids and Gases - science
advertisement
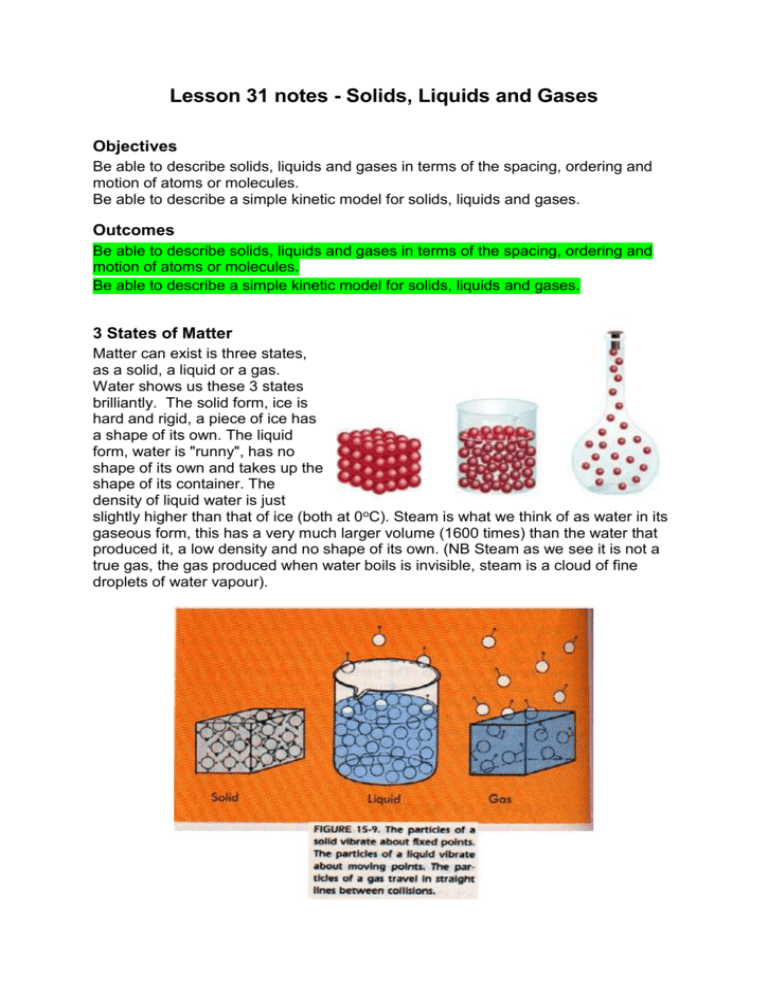
Lesson 31 notes - Solids, Liquids and Gases Objectives Be able to describe solids, liquids and gases in terms of the spacing, ordering and motion of atoms or molecules. Be able to describe a simple kinetic model for solids, liquids and gases. Outcomes Be able to describe solids, liquids and gases in terms of the spacing, ordering and motion of atoms or molecules. Be able to describe a simple kinetic model for solids, liquids and gases. 3 States of Matter Matter can exist is three states, as a solid, a liquid or a gas. Water shows us these 3 states brilliantly. The solid form, ice is hard and rigid, a piece of ice has a shape of its own. The liquid form, water is "runny", has no shape of its own and takes up the shape of its container. The density of liquid water is just slightly higher than that of ice (both at 0oC). Steam is what we think of as water in its gaseous form, this has a very much larger volume (1600 times) than the water that produced it, a low density and no shape of its own. (NB Steam as we see it is not a true gas, the gas produced when water boils is invisible, steam is a cloud of fine droplets of water vapour). Phase Changes The diagram shows phase changes between solid, liquid and gas. Between each phase change there is no temperature change as the bonds are breaking or forming. It also shows Plasma which is an ionized gas normally formed by heating it up. The enthalpy of the system is the heat energy of an object. As molecules inside an object have more enthalpy their vibrations and therefore their kinetic energy goes up. The Kinetic Molecular Theory Particles are in constant motion. In solids the particles are close together and have limited motion. In a liquid some of the attraction between particles is overcome which allows the particles more freedom of movement. In a gas particles attraction between particles is minimized and the particles move freely throughout the container. Standard temperature and pressure (STP on NTP) This set of conditions which is usually applied to gases is defined as a temperature of 273 K and a pressure of 760 mm of mercury (1.013x105 Pa). At STP 1 mole of any gas has a volume of 22.4x10-3 m3. Extension Burning Candles Another example of solids, liquids and gases is a burning candle. Carefully light a candle and watch the surface of the candle and the flame. The flame melts the solid wax, creating a pool of liquid wax. This creeps up the wick inside the flame and becomes a gas, which then burns in the flame. The flame melts more wax and the cycle continues. Sublimation A few compounds go from solid to gas without becoming liquid in between. Solid carbon dioxide (dry ice) becomes carbon dioxide gas without becoming a liquid, as does iodine. In some circumstances, snow and ice can sublime – sometimes, wet washing hung outside freezes solid and then dries without melting. This is called freeze-drying and is used in food preserving and for making instant foods and drinks. Intermolecular Forces In a solid the molecules are held together by intermolecular forces, the exact type of force depending on the type of solid. These forces are divide into four types: (a) ionic bonds - sodium and chloride ions in sodium chloride; (b) covalent bonds - shared electrons between atoms; (c) metallic bonds - free electrons wandering through a metal; (d) Van der Waal's bonds - electric dipole forces Structure of Solids Crystalline solids have specific molecular structures – here are a few examples:








