2006 11 09 Gas chromatography
Chapter 24
Gas Chromatography
Separation Process p. 528 Harris(7 th
) Gas-Iiquid partition chromatography
stationary phase is a non-volatile liquid coated inside the column p. 578 Harris(6 th
) Gas-solid adsorption chromatography
analyte is adsorbed directly on solid particles of stationary phase
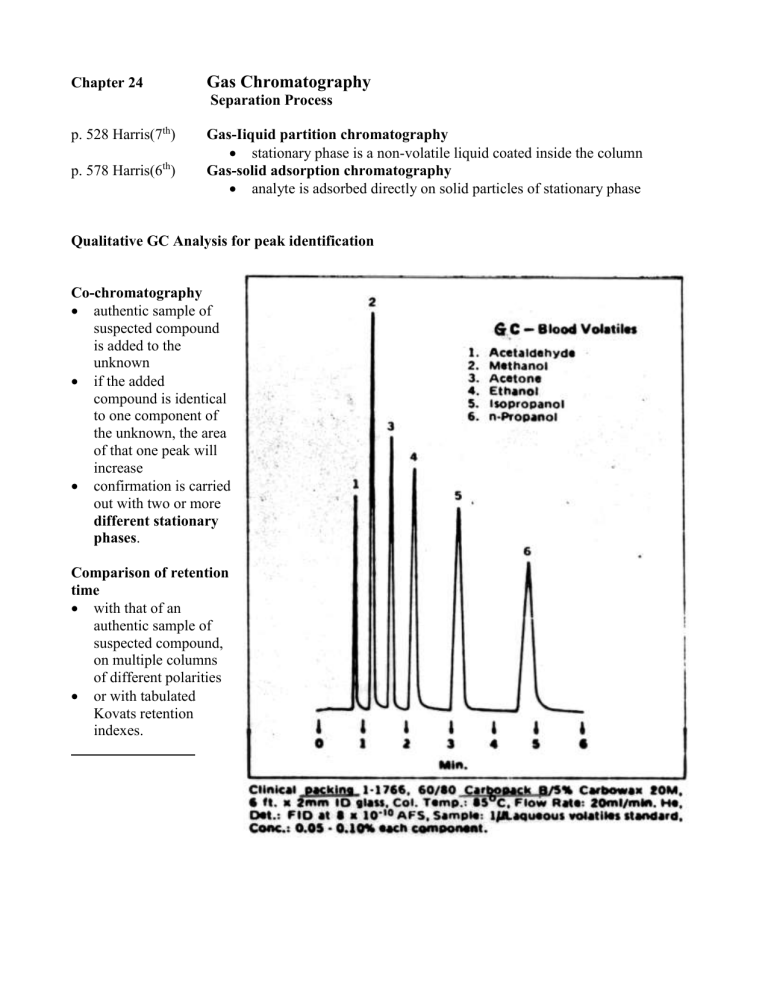
Qualitative GC Analysis for peak identification
Co-chromatography
authentic sample of suspected compound is added to the unknown
if the added compound is identical to one component of the unknown, the area of that one peak will increase
confirmation is carried out with two or more different stationary phases .
Comparison of retention time
with that of an authentic sample of suspected compound, on multiple columns of different polarities
or with tabulated
Kovats retention indexes.
Open tubular columns
Open Tubular Columns p. 529 Harris 7 th p. 579 Harris 6 th
Figure 24-1 Schematic diagram of a gas chromatograph p. 529 Harris 7 th
A volatile or gaseous sample is injected to a heated port.
The sample vapor is carried through the OT column by He, N
2
The choice of carrier gas depends on the detector and…
or H
2
gas.
Question
Why do H2 and He allow more rapid linear flow rates in gas chromatography than does N2 without loss of column efficiency?
Advantages of GC
suited for gaseous samples (with components of different boiling pc
simple and inexpensive equipment (no high-pressure pumps)
rapid chromatography with high resolution (1000-2000 plates /m)
- it is practical to use columns with N = 250,000 -1,000,000 plates
Disadvantages of GC
one interaction phase (liquid/solid stationary phase)
- carrier gas has no interaction with sample components
for low-boiling (M.W. < 300) or volatile (derivatized) organic compounds (not electrolytes)
molecules must be thermally stable
useful for only 15-20 % of all organic compounds
24-1 Sample Injection
Figure 24-13 p. 538 Harris 7 th
Figure 24-10 p. 587 Harris 6 th
Port for split injection into an open tubular column.
Sample is injected through the septum into the sample evaporation zone.
Carrier gas from the flow controller sweeps the sample through silanized glass wool -> complete vaporization and good mixing.
At the split point some of the sample enters the chromatography column and the remainder goes through needle valve 2 to a waste vent and is discarded.
The fraction of sample discarded is controlled by the pressure regulator leading to needle valve 2.
For dirty samples, an adsorbent packing material can be placed inside the liner to irreversibly adsorb undesirable components of the sample.
The glass liner is slowly contaminated by non-volatile and decomposed sample components. It must be replaced regularly.
Open Tubular Columns
Figure 24-2 p. 580 Harris 6 th
Figure 24-2 p. 676 Harris 7 th
(a) Typical dimensions of fused silica open tubular column for GC.
(b) Column lengths are 15-100 m (around the <I> =0.2 m cage).
The Column
Gas chromatographic separation of a perfume oil on Carbowax 20 M as stationary phase:
(upper trace) 1.5-m-Iong x 2-mm-diameter packed column;
(lower trace) 3o-m-Iong x 0.25-mm-diameter open tubular column.
Compared to packed columm, open tubular columm offer/are
much narrower and much longer .
much sharper peaks -> higher resolution. o N/L is increased and column is longer.
shorter analysis time o smaller volume of stationary phase -> solutes are less retained.
greater sensitivity o fluctuations of carrier gas flow (and hence detector response) are damped by the long capillary column.
less sample capacity.
(a) Why do open tubular columns provide greater resolution than packed columns in gas chromatography?
Questions
1. What are the relative advantages and disadvantages of packed and open tubular columns in gas chromatography ?
2.
What is the advantage of a bonded stationary phase in gas chromatography ?
Figure 24-2
(c) Cross-sectional view of wallcoated, support-coated, and porous-layer open tubular columns.
In SCOT, a solid support (silica or alumina) is attached to the inner wall of the column, greatly increasing the available surface area and hence the volume of stationary phase
greater capacity factor
better resolution of poorly retained solutes at the beginning of the chromatogram.
larger samples can be analyzed in trace analysis without saturating the stationary phase.
Sample is injected through the septum into the sample evaporation zone .
Carrier gas from the flow controller sweeps the sample through silanized glass wool -> complete vaporization and good mixing .
At the split point some of the sample enters the chromatography column and the remainder goes through needle valve 2 to a waste vent and is discarded.
The fraction of sample discarded is controlled by the pressure regulator leading to needle valve 2.
p. 539 Harris 7 th p. 587-591 Harris 6 th
Injector temperature
if too low, some components are not completely vaporized. Fractionation of sample occurs, leading to errors in qualitative analysis.
if too high, decomposition of sample can occur, with loss of some components and creation of new species not present in the original sample .
Note: The glass liner and silanized glass wool slowly become contaminated with nonvolatile and decomposed samples. These parts must be replaced periodically.
Column temperature
need not be above the boiling point of all solutes;
must only be hot enough for each solute to have sufficient vapor to be eluted in a reasonable time.
Detector temperature
higher than the column temperature, so that all solutes are gaseous in the detector chamber.
Gas Chromatography
(from http://www.shu.ac.uk/schools/sci/chem/tutorials/chrom/gaschrm.htm)
Introduction
Gas chromatography - specifically gas-liquid chromatography - involves a sample being vapourised and injected onto the head of the chromatographic column. The sample is transported through the column by the flow of inert, gaseous mobile phase. The column itself contains a liquid stationary phase which is adsorbed onto the surface of an inert solid.
Have a look at this schematic diagram of a gas chromato graph :
Instrumental components
Carrier gas
The carrier gas must be chemically inert . Commonly used gases include nitrogen, helium, argon , and carbon dioxide. The choice of carrier gas is often dependant upon the type of detector which is used. The carrier gas system also contains a molecular sieve to remove water and other impurities.
Sample injection port
For optimum column efficiency , the sample should not be too large, and should be introduced onto the column as a "plug" of vapour - slow injection of large samples causes band broadening and loss of resolution . The most common injection method is where a microsyringe is used to inject sample through a rubber septum into a flash vapouriser port at the head of the column. The temperature of the sample port is usually about 50
C higher than the boiling point of the least volatile component of the sample. For packed columns, sample size ranges from tenths of a microliter up to 20 microliters.
Capillary columns, on the other hand, need much less sample, typically around 10 -3
L. For capillary
GC, split/splitless injection is used. Have a look at this diagram of a split/splitless injector;
The injector can be used in one of two modes; split or splitless. The injector contains a heated chamber containing a glass liner into which the sample is injected through the septum. The carrier gas enters the chamber and can leave by three routes (when the injector is in split mode ). The sample vapourises to form a mixture of carrier gas, vapourised solvent and vapourised solutes. A proportion of this mixture passes onto the column, but most exits through the split outlet. The septum purge outlet prevents septum bleed components from entering the column.
Columns
There are two general types of column, packed and capillary (also known as open tubular ). Packed columns contain a finely divided, inert, solid support material (commonly based on diatomaceous earth ) coated with liquid stationary phase. Most packed columns are 1.5 - 10m in length and have an internal diameter of 2 - 4mm.
Capillary columns have an internal diameter of a few tenths of a millimeter. They can be one of two types; wall-coated open tubular (WCOT) or support-coated open tubular (SCOT). Wall-coated columns consist of a capillary tube whose walls are coated with liquid stationary phase. In supportcoated columns, the inner wall of the capillary is lined with a thin layer of support material such as diatomaceous earth, onto which the stationary phase has been adsorbed. SCOT columns are generally less efficient than WCOT columns.
Both types of capillary column are more efficient than packed columns.
In 1979, a new type of WCOT column was devised - the Fused Silica Open Tubular (FSOT) column;
These have much thinner walls than the glass capillary columns, and are given strength by the polyimide coating. These columns are flexible and can be wound into coils. They have the advantages of physical strength, flexibility and low reactivity.
Column temperature
For precise work, column temperature must be controlled to within tenths of a degree . The optimum column temperature is dependant upon the boiling point of the sample. As a rule of thumb, a temperature slightly above the average boiling po int of the sample results in an elution time of 2 - 30 minutes. Minimal temperatures give good resolution, but increase elution times. If a sample has a wide boiling range , then temperature programming can be useful. The column temperature is increased
(either continuously or in steps) as separation proceeds.
Detectors
There are many detectors which can be used in gas chromatography. Different detectors will give different types of selectivity . A non-selective detector responds to all compounds except the carrier gas, a selective detector responds to a range of compounds with a common physical or chemical property and a specific detector responds to a single chemical compound. Detectors can also be grouped into
concentration dependant detectors and mass flow dependant detectors . The signal from a concentration dependant detector is related to the concentration of solute in the detector, and does not usually destroy the sample Dilution of with make-up gas will lower the detectors response. Mass flow dependant detectors usually destroy the sample, and the signal is related to the rate at which solute molecules enter the detector. The response of a mass flow dependant detector is unaffected by make-up gas. Have a look at this tabular summary of common GC detectors:
Detector Type
Support gases
Selectivity Detectability
Dynamic range
Flame ionization
(FID)
Thermal conductivity
(TCD)
Mass flow
Hydrogen and air
Concentration Reference
Mass flow
Hydrogen, oxygen
Most organic cpds.
Universal
Halide, nitrogen, nitrosamine, sulphur
100 pg
1 ng
Electron capture (ECD)
Nitrogenphosphorus
Flame photometric
(FPD)
Photoionization
(PID)
Concentration Make-up
Mass flow
Mass flow
Concentration Make-up
Halides, nitrates, nitriles, peroxides, anhydrides, organometallics
Hydrogen and
Nitrogen, phosphorus air
Hydrogen and Sulphur, phosphorus, tin, air possibly oxygen boron, arsenic, germanium, selenium, chromium
50 fg
10 pg
100 pg
Aliphatics, aromatics, ketones, esters, aldehydes, amines, heterocyclics, organosulphurs, some organometallics
2 pg
Hall electrolytic conductivity
10
10
10
10
10
10
7
7
5
6
3
7
The effluent from the column is mixed with hydrogen and air, and ignited. Organic compounds burning in the flame produce ions and electrons which can conduct electricity through the flame. A large electrical potential is applied at the burner tip, and a collector electrode is located above the flame. The current resulting from the pyrolysis of any organic compounds is measured. FIDs are mass sensitive rather than concentration sensitive; this gives the advantage that changes in mobile phase flow rate do not affect the detector's response. The FID is a useful general detecto r for the analysis of organic compounds; it has high sensitivity, a large linear response range, and low noise. It is also robust and easy to use, but unfortunately, it destroys the sample.
Review your learning
You should be aware of how a GC instrument works and the principles behind the operation of the major instrumental components, including injectors, columns and detectors.






