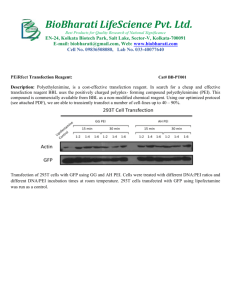
Large scale Lentivirus production
advertisement

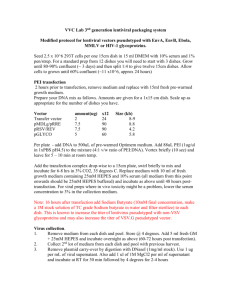
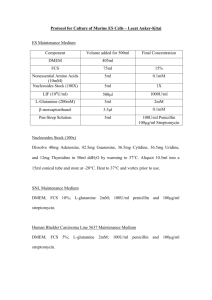
Large scale lenti virus production (transfection with PEI) (Based on “Tiscornia G, Singer O, and Verma I. Nature Protocols, 2006”) Seed HEK293FT cells on 14x150mm (145cm2), pre-coated with poly-l-lysine. plates, transfect when 80-90% confluent. (Not more than 90% confluent, to allow another cell division). Use cells in passage lower than 20. HEK293FT were developed by Invitrogen for better adherence, HET293T can also be used, and improved adherence can be acheaved by coating the plates with either gelatin or polylysine, using standard procedures. Transfection: The 3 tables are examples of 3 types of viruses, available from ADDGENE Prepare DNA mix according to the desired Virus: 2nd generation lentivirus Plasmid Transfer (e.g FCGW) Packging - Δ8-9 Envelope - VSVG ug [stock] vol x14 (μl) 10 10 6. 7 3rd generation lentivirus Plasmid Transfer (e.g. pCSC) Packaging pMDL Packaging pREV Envelope VSVG ug [stock] vol x14 (μl) 12 7.8 3 4.2 1. Add total of 378ug DNA into 16.8 ml of DMEM without serum. 2. Add 504ul of 1 mg/ml PEI (Diluted from 10mg/ml stock1 with sterile DDW). 3. Vortex briefly. 4. Incubate 10 min at RT. 5. During incubation, rinse the cells carefully with serum free DMEM, and then add 11 ml DMEM (no serum) to each plate. 1 PEI stock solution preparation: Use Polyethyleneimine, high molecular weight, water free (Aldrich, cat #408727). Prepare 10 mg/ml stock – weigh the PEI solution and dissolve in DDW, sterilize with 0.2um filter, aliquot and keep in -80oc. Don’t reuse thawed PEI solution. 6. After the 10 min incubation, add 1.23ml DNA/PEI mix to each plate (Drops). 7. After 3-4h, add 1.3 ml of 100% FBS. Alternatively, incubate overnight without serum, and the morning after change to 13ml of full growing medium (DMEM, 10% FBS, PSA and L-glutamnie). Virus collection and concentration: 1. Collect conditioned medium ~36h post transfection 2. Pre-wet 0.45um filter (Durapore) with 20ml DMEM + 10% serum. Discard the medium, filter the conditioned media, and keep in 4oc 3. Re-feed the cells again with complete medium and place in incubator for extra 24h. 4. Wash conical ultracentrifuge tubes and the SW-28 buckets, adapters and plugs with 70% ethanol, dry well with sterile kim-wipes. 5. Load 6x conical ultracentrifuge tubes with the 1st collection, use SW28 rotor. (load 33-34ml each tube, inside the hood ,weigh on scales inside the hood). 6. Spin in Beckman ultracentrifuge: 20,000rpm, 2:00h, 15oc 7. During first centrifugation, pre-wet 0.45μm filter with 20ml DMEM + 10% serum. Discard the medium, and filter the 2nd round of conditioned media. 8. When spin is over, pour the medium into bio-hazard vessel with bleach, load the 2nd round into the same 6 tubes, and ultracentrifuge 20,000rpm, 2:00h, 15oc. 9. Pour the medium (into biohazard vessel with bleach) 10. Resuspend in 250ul PBS, and transfer sequentially into 6 tubes. Avoid bubbles (use large tips). 11. Collect the total of concentrated virus into a single tube, wrap in parafilm and incubate in rotation shaker in cold room for 15’-30’. Spin 10s. 12. Aliquot 4ul of virus into each tube (label tubes before!). 13. Wash all the tubes, buckets and adapters with bleach and than a lot of water. Virus titer determination: 1. Make ten fold serial dilution of the virus preparation in PBS. 2. Seed cells in a 24 well plate (~50,000 cells per well) in 500ul DMEM + 10%FBS. 3. Immediately after seeding, add 50ul of each dilution (additional 10 fold dilution) to the medium (before the cells adherence to the plate). Check from 10-3 to10-8. 4. Incubate the cells 48h, and check fluorescence. The approximate titer is the highest dilution where fluorescence is still visible. Virus titer__________________