Table 2: Summary of the design, methodology
advertisement
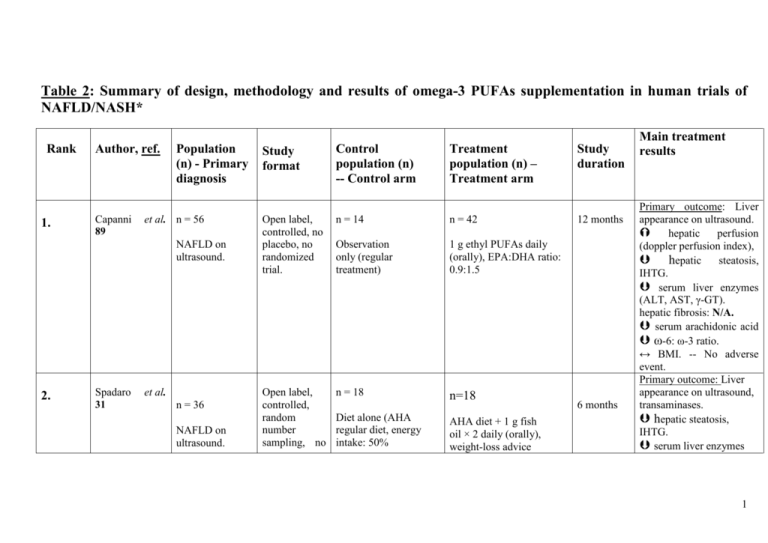
Table 2: Summary of design, methodology and results of omega-3 PUFAs supplementation in human trials of NAFLD/NASH* Rank 1. Author, ref. Capanni 89 Population (n) - Primary diagnosis et al. n = 56 NAFLD on ultrasound. 2. Spadaro 31 et al. n = 36 NAFLD on ultrasound. Study format Control population (n) -- Control arm Treatment population (n) – Treatment arm Open label, controlled, no placebo, no randomized trial. n = 14 n = 42 Observation only (regular treatment) 1 g ethyl PUFAs daily (orally), EPA:DHA ratio: 0.9:1.5 Open label, controlled, random number sampling, no n = 18 n=18 Diet alone (AHA regular diet, energy intake: 50% AHA diet + 1 g fish oil × 2 daily (orally), weight-loss advice Study duration 12 months 6 months Main treatment results Primary outcome: Liver appearance on ultrasound. hepatic perfusion (doppler perfusion index), hepatic steatosis, IHTG. serum liver enzymes (ALT, AST, γ-GT). hepatic fibrosis: N/A. serum arachidonic acid ω-6: ω-3 ratio. ↔ BMI. -- No adverse event. Primary outcome: Liver appearance on ultrasound, transaminases. hepatic steatosis, IHTG. serum liver enzymes 1 n =23 3. Tanaka et al. 90 Lliver biopsy proven NASH patients. placebo, single-blinded trial. carbohydrates, 20% protein, 30% fat), weight-loss advice. (EPA+DHA: >85% of PUFAs, ratio: 0.9–1.5) Open label, no randomized, no placebo trial. n = N/A n= 23 Control arm: N/A 2.7 g highly (>98%) purified EPA-ethyl ester, daily. 12 months (ALT, γ-GT), serum TNFα. ↔ AST. HOM-IR. serum HDL. hepatic fibrosis: N/A. BMI decreased to similar extents in both groups. -No adverse event. Primary outcome: liver appearance on ultrasound, liver histology. hepatic steatosis (relative to baseline). liver enzymes ALT, AST. HOMA-IR. ↔ γ-GT. lobular inflammation, ballooning. NAS and fibrosis score dropped ≥ 1 in 6/7 patients (85%). Serum free FAs, total cholesterol, AA, ferritin, thioredoxin. Plasma sTNF-R1, sTNFR2 levels -- No adverse event. 2 4. Vega et al. 95 n=16 NAFLD on magnetic resonance spectroscopy (MRS). n=52 5. Itoh et al. 60 Obesity. n=144 6. F.S. Zhu et al 96 NAFLD with hyperlipidemia Open label, no randomized, no placebo, cross-over design. n=N/A n= 16 No control arm 9 g of fish oil daily. Single blind, randomized, no placebo trial. n=26 n=26 Dietary advice only. 1.8 g EPA daily (highly purified EPA- ethyl ester, purity >98%). Randomized, placebocontrolled, triple-blind trial. n = 72 n = 72 2 g placebo × 3 daily, 6 AHA diet + weight loss months + weight loss advice + 2 g seal advice. oil × three times a day, for 6 months (520 mg ω-3: Primary outcome 1 month of measures: Plasma and hepatic triglycerides. placebo serum triglyceride level. followed by 2 Hepatic fibrosis: N/A. months of ↔ liver fat content. PUFA treatment. 3 months 6 months Primary outcome: Serum adiponectin. Hepatic fibrosis: N/A. plasma triglycerides. serum adiponectin. hepatic steatosis. ALT. ↔ AST, γ-GT. NASH score: N/A. Adverse events: No difference btw groups. TG. NAFLD symptom score, AST, γ-GT, total and LDL cholesterol. 3 EPA 175 mg, DPA 235 mg, DHA 110 mg). 7. 8. Mario Kratz et n = 26 al 98 Obesity. Sofi F. et al 99 n = 11 NAFLD on ultrasound Randomized, controlled, no placebo, dietary intervention trial. Randomized, placebocontrolled, dietary intervention trial. HDL cholesterol (to similar extents in both groups). 3.5 months n = 13 n = 13 Control diet (rich in monounsaturated fatty acids typically mixed American diet, consisting of 0.5% of energy intake from ω– 3 PUFAs). Control diet for the first two weeks, than diet rich in ω–3 PUFAs (3.5% of energy intake) from both plant and marine sources. total plasma adiponectin. ↔ HMW plasma adiponectin and HMW: total plasma adiponectin ratio in both diet groups. ↔ fasting glucose, insulin concentrations, HOMA-IR index, diurnal adiponectin amplitude. 12 months n=5 n=6 Dietary recommendations + olive oil not enriched with ω-3 PUFAs. Dietary recommendations + daily oral administration of 6.5 ml olive oil enriched with 0.83 g omega-3 PUFAs (0.47 g EPA + 0.24 g DHA). plasma liver enzymes (AST, ALT and GGT). serum triglycerides. serum adiponectin levels. HDL. Improvement of liver ultrasonography. 4 2 months 9. 10. Cussons A.J. et n = 25 101 al Obese premenopaused women with PCOS Doubleblinded, placebocontrolled trial with a randomized cross-over design. Control (placebo) diet: 4 × 1000 mg capsules of olive oil containing 67% oleic acid, daily. Hatzitolios et al 102 Uncontrolled design, no randomized, no placebo trial. Alternative lipidn = 73 lowering pharmacological agents 13.7 g omega-3, daily. (orlistat, atorvastatin) n = 73 Mixed dyslipidemia, persistent transaminasemia, hepatic fat infiltration on ultrasonography and liver biopsy. n = 25 Primary outcome measure: hepatic fat content (quantified by proton MRS). 4g daily marine-derived omega-3 PUFAs (56% DHA + 27% EPA) liver fat content, serum triglycerides, blood pressure. 6 months serum transaminases. Normalization of ultrasonographic evidence of fatty liver. 5 6 months 11. Nobili V. et al. n = 60 children 103 or adolescents (4-16 years) Biopsy-proven NAFLD. Randomized, placebocontrolled, double-blind interventional study. n = 20 n = 40 Placebo (290 mg linoleic acid supplied with germ oil). DHA supplementation: Main outcome measures: liver fat content. (by ultrasonography). Similarly in both DHA groups: insulin sensitivity index serum triglycerides. n = 20 (250 mg/day) and n = 20 (500 mg/day) (39% DHA oil obtained from Schyzochitrium ↔ ALT and body mass index. 12. H.M. Parker et n=355 88 al NAFLD/NASH patients. Meta-analysis of data from 9 eligible trials ** (4 RCTs, 1 randomised placebo controlled cross-over design, 1 quasi experimental cross-over Control group: 1 quasi experimental design trial (all participants underwent omega-3 treatment + those who refused treatment) n = 355 Median ω-3 PUFA dose = 4 g/day (range: 0.83– 13.7 g/day). Median duration of treatment with omega-3 PUFAs: 6 months (range: 212 months). Primary outcome measures: liver fat and function tests (ALT, AST). liver steatosis (quantified by ultrasound, magnetic resonance spectroscopy or needle biopsy). Benefits are seen with more than 0.83 g/day of omega-3 supplementation No significant effect on 6 design, 2 uncontrolled design, all participants underwent omega-3 treatment. liver tests. A no significant trend favouring PUFA treatment on ALT. No reports of adverse effects. * Clinical trials conducted between 2004 – 2012. Abbreviations: AA: arachidonic acid; AHA: American Heart Association; ALT: alanine aminotransferase; AST: aspartate aminotransferase; BMI: body mass index; FAs: fatty acids; γ-GT: gamma-glutamyl-transferase; GGT: g-glutamyl-transpeptidase; HDL: high-density lipoprotein; HMW: high-molecular weight; HOMA-IR: homeostatic model assessment of insulin resistance; IHTG: intra-hepatic triglyceride; MRS: magnetic resonance spectroscopy; NAS: NAFLD Activity Score; PCOS: polycystic ovary syndrome; sTNF-R: soluble tumor necrosis factor- receptor; TNF-α: tumor necrosis factor-alpha; 7