Exam 2 CHEMISTRY 1A
advertisement

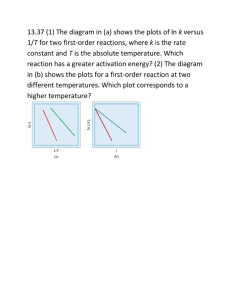
Exam 4 Ch 14, 15 CHEMISTRY 1B 100 Points Name______________________________ SHOW YOUR WORK FOR FULL CREDIT!!!! 1. A solution contains 795 grams of K3PO4 and 3.35 kilograms of water. For this solution calculate: (20) a. the mass percent K3PO4. b. the mole fraction K3PO4. c. the molality. d. the boiling point. ( Kbp = 0.512 o/molal ) e. the vapor pressure at 76.oC. ( Po= 301 torr ) 2. Calculate the molar mass of a compound if a solution formed by dissolving 1.45 grams of compound in 7.85 grams of cyclohexane, C6H12, has a boiling point of 84.48C. Cyclohexane has a bp = 80.74C. Kbp = 2.79 C/molal. (10) 3. Calculate the enthalpy of solution for RbF given the enthalpy of formation for the solid, -557.7 kJ/mole and for the solution,-583.8 kJ/mole. Include the chemical reaction equation and explain what happens to the molar solubility of the salt when the temperature is increased. (5) 4. A solution of a water soluble vitamin is prepared by dissolving 14.35 mg of the compound in enough water to form 25.00 mL of solution at 33C. The osmotic pressure is 62.00 torr. Calculate the molar mass of the compound and its molecular formula, if the compound is 4.58% H, 54.5% O, and 40.9% C. (15) 5. At 40.0 C the vapor pressure of pure chloroform, CHCl3 is 415 torr and of pure carbon tetrachloride, CCl4 is 275 torr. Calculate the mole fraction of chloroform: (15) a. in a solution with CCl4 at 40. C if the total pressure is 330. torr. b. in the vapor state above the solution described in a) above. 6. Complete the table below for the reaction Q Products: differential form integrated form half-life expression (12) sketch for straight line plot Second-order First-order Zero-order 7. The reaction below is first-order in H2 and second-order in NO. (8) 2 H2(g) + 2 NO(g) N2(g) + 2 H2O(g) a. Write the rate law in terms of the disappearance of H2. b. What is the overall order of the reaction? c. Using related rates, write the rate of formation of N2 in terms of the rate of disappearance of NO. 1 8. Draw a reaction diagram (Energy vs Time) for a reaction with the activation energy for the forward reaction being 45 units, the activation energy for the catalyzed reaction being 20. units less than that for the uncatalyzed reaction, and the activation energy for the reverse catalyzed reaction being 15 units. Calculate the activation energy for the catalyzed reaction, the reaction energy, and the activation energy for the uncatalyzed reverse reaction. Label the diagram completely!! Is the reaction endothermic or exothermic? (8) 9. The decomposition of PH3 proceeds according to the equation: 4 PH3(g) P4(g) + 6 H2(g) with t1/2 = 0.75 minutes. The reaction is first-order with respect to the PH3. Calculate: a. the rate constant in sec-1. b. the time required for 7/8 of the PH3 to decompose starting with 0.80M PH3. c. the rate of appearance of H2 when [PH3] = 0.80M. (10) 10. 2 NO(g) + O2(g) 2 NO2(g) [NO]o M [O2]o M Initial Rate of NO2 formation M/min. (18) a. b. c. d. 0.010 0.020 1.4x10-2 0.010 0.070 5.0x10-2 0.040 0.070 7.9x10-1 Deduce the rate law. SHOW YOUR WORK!!!! Determine the over all order. Calculate the rate constant. Calculate the reaction rate when the initial concentrations are 0.025M for NO and 0.045M for O2. 11. For a certain reaction the activation energy is 38.0 kJ/mole. Calculate the percent increase in the rate constant, k, when the temperature is increased from 132C to 187C . 12. For the reaction 2 O3(g) 3 O2(g) the following mechanism is proposed: Fast K O3(g) <=====> 3 O(g) Slow k1 O3(g) + O(g) _________> 2 O2(g) (7) (12) a. Is the mechanism consistent with the stoichiometry? HOW? b. Write the rate law in terms of the rate-determining step. c. Write the rate law in the form: rate = k [O3]x , where k and x are determined from the mechanism. 2