N - Molecular Compounds
advertisement

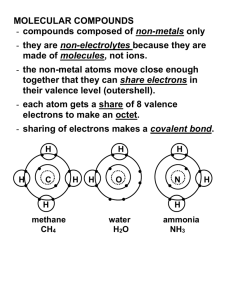
2.7 Molecular Compounds Not all chemicals are ionic compounds (composed of a metal and a non-metal) Some atoms don’t exchange electrons but share them to form neutral groups of atoms called molecules (molecular compounds) i.e sugar, water, carbon dioxide etc. Molecular Compounds: 2 non metals share electrons to form stable arrangements. *Atoms are joined by covalent bonds. Covalent Bond: the force of attraction between atoms that share one, two, or three pairs of electrons. Sharing one pair of electrons single bond Sharing 2 pairs of electrons double bond Sharing 3 pairs of electrons triple bond Some characteristics of covalent molecules: force of attraction between molecules is weak; have relatively low melting points since little energy is needed to break the force of attraction between molecules; tend not to conduct electricity since they don’t contain ions. Example of covalent molecule Cl2 A chlorine atom has 7 electrons in its outer shell so it needs 1 more electron to be stable (this make chlorine very reactive!!) Two chlorine atoms share a pair of electrons to form Cl2 – a diatomic molecule (di- means two). Practice a) 02 dioxide b) F2 difluoride Other example of diatomic (covalent) molecules: Name of element Chemical Symbol Hydrogen Oxygen Nitrogen Fluorine Chlorine Bromine Iodine H O N F Cl Br I Formula (and state at room temp) H2 (gas) O2 (gas) N2 (gas) F2 (gas) Cl2 (gas) Br2 (liquid) I2 (solid) More examples of covalent molecules: H H2 H hydrogen gas H CH4 methane H C H H H O H2O water H Writing Formulas for Molecular Compounds The criss-cross rule is used in the exact same way we used it for ionic compounds Step 1. Write the symbols, starting with the element further left (on the Periodic Table) with the number of electrons needed to fill their outer shells. 4 2 C S Step 2. Crisscross to produce subscripts. 4 2 C S C 2S4 Step 3. Do NOT reduce the formula….leave AS IS!!! But do not reduce the subscripts for molecular compounds!!! Formula C 2 S4 CS2 Write the formulas for the following: a) sulfur and oxygen b) carbon and fluorine Naming Molecular Compounds The system of naming for molecular compounds however is much different than for ionic compounds. Prefixes are used when naming molecular compounds. Prefix mon(o) ditritetrapent(a) Number 1 2 3 4 5 Step 1 Write the name of the first element (the one farthest to the left) with its proper prefix. *IF THERE ARE ONLY ONE ATOM THEN DO NOT WRITE MONO i.e. CO carbon monoxide (not monocarbon monoxide) Step 2 Write the name of the second element (the one farthest to the left) with its prefix. Practice a) CBr4 __________________________________ b) NI3 __________________________________ c) OF2 __________________________________ d) SiCl4 __________________________________ Molecular Compounds Exercise Name: _____________________________ Date: _____________________________ 1. What kinds of atoms form molecular compounds? 2. How do the atoms in molecular compounds form stable electron arrangements? 3. What type of bond holds atoms together in molecules? 4. a) How many valence electrons are there in a fluorine atom? __________ b) How many electrons does a fluorine atom need to share to become stable? _________ c) Draw a diagram to show how two fluorine atoms could form a stable molecule. 5. Name the following compounds (use prefixes): a) SiCl4 b) PI3 c) SF2 d) NBr4 6. Write the chemical formulas and name of the compounds formed by the following pairs of elements. a) silicon and oxygen b) nitrogen and hydrogen c) phosphorus and chlorine d) sulfur and bromine e) oxygen and fluorine