Supplementary Information (doc 550K)
advertisement

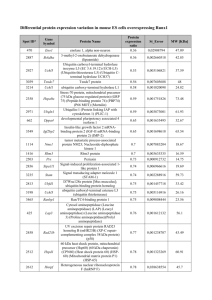
Supplementary Information Additional details on the patient cohorts .......................................................................... 2 Next-generation deep-sequencing target accession numbers (NGS) ............................. 4 PCR primer design for next-generation deep-sequencing............................................... 4 PCR amplification protocols for next-generation deep-sequencing ................................. 6 PCR amplification 96-well plate layout ............................................................................ 7 Assay development phase .............................................................................................. 8 1 Additional details on the patient cohorts The molecular deep-sequencing RUNX1 mutation analysis was established using a retrospective pre-characterized proof-of-principle cohort of 24 patients with 105 serial samples available (Supplementary Figure S1a). Subsequently, starting 07/2010 and ending 04/2012, an independent 814 AML cases were prospectively investigated as part of routine diagnostic procedures. For additional serial analyses, 57 AML cases with detectable RUNX1 mutations were studied at diagnosis and relapse. A prognostic model was investigated using an independent cohort of 103 AML cases diagnosed between 08/2005 – 05/2012 with additional samples obtained during course of therapy. Regarding the overlap of the distinct patient cohorts a Venn diagram is shown in Supplementary Figure S1b. The prospective cohort of 814 cases did not overlap with the assay development cohort of 24 cases. However, there were 10 cases overlapping between the prospective cohort of 814 cases and the 57 patients studied to assess the stability of RUNX1 mutations between diagnosis and relapse. 44 cases were overlapping between the prospective cohort of 814 cases and the 103 patients studied to assess the prognostic impact of residual RUNX1 mutations after treatment with AMLspecific intensive treatment protocols. Nine patients were overlapping across all three cohorts. 2 (a) Stability Model: 57 paired AML samples from diagnosis and relapse Prognostic Model: 103 AML cases with clinical follow-up Assay Development Phase • • • • 24 patients 18 AML and 6 MDS 105 serial samples obtained 11/2005 - 04/2010 Prospective Testing 07/2010 – 04/2012 (22 months) 814 AML cases at diagnosis mutational landscape analysis of RUNX1 mutations (b) Supplementary Figure S1 (a) Overview on the distinct patient cohorts during the assay development, longitudinal analyses, and prospective testing phases for RUNX1 mutation analysis. (b) Venn diagram depicting the overlap between cohorts. A: cohort of 814 prospective AML cases; B: cohort of 57 paired AML samples to assess RUNX1 mutations at diagnosis and relapse; C: cohort of 103 cases to study prognostic impact of residual RUNX1 mutations. 3 Next-generation deep-sequencing target accession numbers (NGS) The primer design for sequencing of genomic DNA was based on the Ensembl accession number given in Supplementary Table S1 (Ensembl 53: Mar 2009). Supplementary Table S1 Ensembl accession numbers for deep-sequencing Gene symbol Ensembl ID Transcript ID RUNX1 ENSG00000159216 ENST00000344691 PCR primer design for next-generation deep-sequencing Supplementary Table S2 lists the corresponding primer pairs for 7 amplicons representing RUNX1. The median amplicon size was 342 bp (range 341 – 348 bp). According to the “Guidelines for Amplicon Experimental Design” by the manufacturer, the 5’-portion was a 25-mer whose sequence is dictated by the requirements of the 454 Sequencing System for binding to the DNA Capture Beads (Lib-A), and for annealing the emPCR Amplification Primers and the Sequencing Primer (Roche Applied Science). This 5’-part also ended with the sequencing key “TCAG” used for amplicon sequencing. We applied two kinds of such primers, termed “Primer A” and “Primer B”, allowing for the directional sequencing of the target sequence from either end: primer A fusion sequence: 5' CGT ATC GCC TCC CTC GCG CCA TCA G 3' primer B fusion sequence: 5' CTA TGC GCC TTG CCA GCC CGC TCA G 3' Distinct molecular barcode sequences were incorporated into the primer sequences after the TCAG sequencing key and before the gene-specific sequence (Figure S2A). 4 Supplementary Table S2 Information on PCR primer and amplification protocols Gene Strand Exon RUNX1 - RUNX1 Amplicon 1 Forward Sequence 5' -> 3' GCTGTTTGCAGGGTCCTAAC Reverse Sequence 5' -> 3' GGCCTCCGCCTGTCCTC Length 348 - 2 CATTGCTATTCCTCTGCAACC GTTTGTTGCCATGAAACGTG 342 RUNX1 - 3 AAATTCCGGGAGTGTTGTCA GAAAGGTTGAACCCAAGGAA 341 RUNX1 - 4 TGATCTCTTCCCTCCCTCCT CAGTTGGTCTGGGAAGGTGT 348 ATTTGAACAAGGGCCACTCA AATGTTCTGCCAACTCCTTCA 342 RUNX1 - 5 RUNX1 - 6 6.01 CTCCGCAACCTCCTACTCAC CCCACCATGGAGAACTGGTA 342 RUNX1 - 6 6.01 CCCGTTCCAAGCCAGCTC GCTTGTCGCGAACAGGAG 342 Notes: Exon, referring to Transcript ID ENST00000344691 the exons are numbered accordingly 1 to 6. However, historically the numbering could also be exons 3 to 8. Amplicon, ascending order of PCR products that are overlapping in an exon Length, base pairs including the sequence-specific primer 5 PCR amplification protocols for next-generation deep-sequencing PCR protocols were performed with ~40 ng template genomic DNA. All PCR master mixes were prepared according to the manufacturer’s recommendations using the FastStart High Fidelity PCR Kit for exons 2, 3, 4, and 5, and using the GC-RICH PCR System kit for GC-rich exons 1 and 6 (Roche Applied Science, Mannheim, Germany). PCR reactions were performed using a 96-Well GeneAmp® PCR System 2720 or 9700 instrument (Applied Biosystems, Foster City, CA) including a final cooling step at 4°C after final elongation (Supplementary Table S3). Supplementary Table S3 PCR amplification protocol Step Cycles Time (min) Temperature Cycles Time (min) Temperature Initial Denaturation 1 5:00 95.0°C 1 5:00 95.0°C Denaturation Annealing Elongation 10 0:30 0:30 0:30 95.0°C 63.0°C 58.0°C 72.0°C 25 0:30 0:30 0:30 95.0°C 58.0°C 72.0°C 1 7:00 72.0°C Final Elongation 6 PCR amplification 96-well plate layout Preconfigured GS GType RUNX1 Primer Set 96-well plates allowed single-plex parallel PCR reactions of up to 12 patients including 12 different MIDs (Roche Applied Science). After purification and quantification the resulting amplicon products were pooled and the pools subsequently diluted to a concentration of 1 x 109 molecules/µl (Supplementary Figure S2B). Figure S1 A 96-well plate format with pre-configured primers (MID) B 12 Patients / plate 1 A B C D E F G H 2 3 4 5 6 7 8 9 10 11 12 RUNX1_TM1_E04 RUNX1_TM2_E04 RUNX1_TM3_E04 RUNX1_TM4_E04 RUNX1_TM5_E04 RUNX1_TM6_E04 RUNX1_TM8_E04 RUNX1_TM10_E04 RUNX1_TM13_E04 RUNX1_TM14_E04 RUNX1_TM15_E04 RUNX1_TM16_E04 FastStart RUNX1_TM1_E05 RUNX1_TM2_E05 RUNX1_TM3_E05 RUNX1_TM4_E05 RUNX1_TM5_E05 RUNX1_TM6_E05 RUNX1_TM8_E05 RUNX1_TM10_E05 RUNX1_TM13_E05 RUNX1_TM14_E05 RUNX1_TM15_E05 RUNX1_TM16_E05 FastStart RUNX1_TM1_E06 RUNX1_TM2_E06 RUNX1_TM3_E06 RUNX1_TM4_E06 RUNX1_TM5_E06 RUNX1_TM6_E06 RUNX1_TM8_E06 RUNX1_TM10_E06 RUNX1_TM13_E06 RUNX1_TM14_E06 RUNX1_TM15_E06 RUNX1_TM16_E06 FastStart RUNX1_TM1_E07 RUNX1_TM2_E07 RUNX1_TM3_E07 RUNX1_TM4_E07 RUNX1_TM5_E07 RUNX1_TM6_E07 RUNX1_TM8_E07 RUNX1_TM10_E07 RUNX1_TM13_E07 RUNX1_TM14_E07 RUNX1_TM15_E07 RUNX1_TM16_E07 RUNX1_TM1_E03 RUNX1_TM2_E03 RUNX1_TM3_E03 RUNX1_TM4_E03 RUNX1_TM5_E03 RUNX1_TM6_E03 RUNX1_TM8_E03 RUNX1_TM10_E03 RUNX1_TM13_E03 RUNX1_TM14_E03 RUNX1_TM15_E03 RUNX1_TM16_E03 GC-rich RUNX1_TM1_E08.1 RUNX1_TM2_E08.1 RUNX1_TM3_E08.1 RUNX1_TM4_E08.1 RUNX1_TM5_E08.1 RUNX1_TM6_E08.1 RUNX1_TM8_E08.1 RUNX1_TM10_E08.1 RUNX1_TM13_E08.1 RUNX1_TM14_E08.1 RUNX1_TM15_E08.1 RUNX1_TM16_E08.1 GC-rich RUNX1_TM1_E08.2 RUNX1_TM2_E08.2 RUNX1_TM3_E08.2 RUNX1_TM4_E08.2 RUNX1_TM5_E08.2 RUNX1_TM6_E08.2 RUNX1_TM8_E08.2 RUNX1_TM10_E08.2 RUNX1_TM13_E08.2 RUNX1_TM14_E08.2 RUNX1_TM15_E08.2 RUNX1_TM16_E08.2 RUNX1_TM14_E07 RUNX1_TM14_E08.2 MID 1 MID 2 MID 3 MID 4 MID 5 MID 6 MID 8 MID 10 MID 13 MID 14 MID 15 MID 16 FastStart GC-rich control pool of amplicons Supplementary Figure S2 Amplicon and library preparation. (A) Principle of a fusion primer setup for 454 amplicon deep-sequencing. Multiplex Identifiers (MIDs) as provided by the manufacturer were used to assign a molecular barcode to the amplicons and samples. (B) Layout of the preconfigured RUNX1 primer plate. After PCR amplification, individual amplicons were purified and pooled. 7 Assay development phase The RUNX1 next-generation amplicon deep-sequencing assay had been developed using a pre-characterized cohort of 24 patients (18 AML and 6 MDS cases), diagnosed between 11/2005 and 04/2010). For each of these patients, one or more molecular RUNX1 mutations were known from standard routine testing using a combination of denaturing high-performance liquid chromatography (DHPLC) and direct capillary Sanger sequencing. This assay development cohort was planned as longitudinal analysis, i.e. samples were analyzed starting at the time point of diagnosis and also included serially collected specimens following the course of treatment. In total, 105 samples from peripheral blood (n=19) or bone marrow specimens (n=86) were collected and analyzed in parallel by DHPLC/direct capillary Sanger sequencing and nextgeneration amplicon deep-sequencing. The 24 patients were investigated with high sequencing coverage, i.e. in median 3,498 reads per amplicon were generated. As highlighted in Supplementary Data Spreadsheet 2 a total of 157 comparisons were made across 105 time points collected for the 24 patients: (i) the NGS analyses concordantly detected the mutations known from previous testing with conventional Sanger sequencing method in 53/53 (100%) comparisons. (ii) in 80/80 (100%) comparisons both methods consistently did not detect any RUNX1 mutation. (iii) Importantly, in the remaining 21 comparisons (13.4%) a discrepant result was observed. In all 21/157 comparisons low-level variants were detected using the amplicon deepsequencing assay. In these patients residual clones still were detectable after treatment had been initiated or NGS detected a change in mutation load earlier than using conventional methods. As highlighted in Supplementary Figure S3, deep-sequencing also allowed to closely monitoring the composition of distinct subclones and their clonality during course of disease. Of note, three comparisons were not performed since in these analyses only the next-generation sequencing assay had provided results while the DHPLC/direct capillary Sanger sequencing workflow had failed to produce results. 8 Supplemental Figure S3 RUNX1 Assay development phase. Early detection of an increasing clone size by deep-sequencing. In this exemplary case (patient #24; Supplementary Data Spreadsheet 2), over the course of disease 4 distinct variants in RUNX1 were detectable. Across the 6 time points that were investigated each of the four variants is given. The deep-sequencing assay allows monitoring a changing composition of clones. On the right y-axis, the number of reads is indicated (grey bar graphs), on the left y-axis, the mutation load, i.e. the percentage of reads harboring the mutation (red bar graphs) is given, respectively. 9