Request_form_NGS_MiSeq_MMC_eng
advertisement

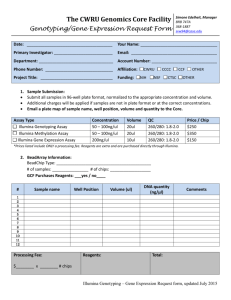
MEDICAL UNIVERSITY– SOFIA 2 Zdrave St., 1431 Sofia, Bulgaria; Tel: (+359) 2 9172 214; Fax: (+359) 2 9172 214 http://www.mmcbg.org; mmcbg@abv.bg Illumina MiSeq Next Generation Sequencing Service Sample Submission Form I. APPLICANT INFORMATION Name:__________________________________________________________________ Date of submission: _______________________________________ Organization:_____________________________________________________________ Department:______________________________________________________________ Invoice/project number:____________________________________________________ Address: _________________________________________________________________ Phone: Email:__________________________ II. SAMPLE INFORMATION Sample ID: ____________________________________________________ Male Sex: Female Sample (double stranded genomic DNA) origin: Blood Tissue Other: ______ Dissolved in TE Buffer Other: ___________ In pure ethanol Sample condition: Dissolved in water Dry powder Sample concentration in ng/ul ______________________ Total sample amount in ng: _____________________________ We will provide reagents and consumables Yes No If yes, we need support in ordering reagents and consumables Yes No If no, we need you to order the reagents and consumables Yes No Please indicate clinical diagnosis, symptoms and family history: ______________________________________________________________ ______________________________________________________________ ______________________________________________________________ ______________________________________________________________ Have any genetic tests already been performed? Yes No If yes, which? Results? ______________________________________________________________ ______________________________________________________________ ______________________________________________________________ ______________________________________________________________ III. TEST REQUESTED Illumina TruSight One Sequencing Panel (Targeting 4,813 genes associated with human diseases) Illumina TruSight Inherited Diseases Sequencing Panel (Targeting 552 genetic regions known to contain pathogenic mutations in severe, recessive, pediatric-onset diseases) Illumina TruSight Cancer Sequencing Panel (Targeting 94 genes suspected to play a role in predisposing to various cancers and 284 SNPs suspected to be associated with cancer through genome-wide association studies) Illumina TruSeq Cancer Sequencing Panel (Targeting 48 cancer hotspot mutations in selected genes/somatic mutations) Yes Bioinformatics analysis needed No If no, what kind of raw data fails are requested? FastQ file BAM file Direct sequencing validation needed VCF file Yes No IV. SAMPLE REQUIREMENTS: Tubes should be labelled with a unique ID and should be submitted with a typed sample information document. Input DNA required for each Illumina TruSight Sequencing Panel is 50ng (preferred 10ng/ul), quantified by a Qubit or qPCR. Input DNA required for Illumina TruSeq Sequencing Panel is 250ng (preferred 50ng/ul), quantified by a Qubit or qPCR. It is recommended to use high quality non-degraded DNA (A260/A280>1,7) diluted in ddH2O. Please provide the total amount of DNA supplied, the concentrations of DNA in ng/ul the method used and the analysis results measured by gel electrophoresis graph. V. INVOICING INFORMATION Person:_________________________________________________________________ Institute:_______________________________________________________________ Department:____________________________________________________________ Address:_______________________________________________________________ Invoice/project number:____________________________________________________ For more information required: About the tests, Help how to choose the right test for each separate case or type of project To discuss the required reagents and consumables for the experiments, To discuss budget issues, please contact To schedule experiments in time To discuss future project design Terms and conditions of using the Genomics Core facilities Please contact with: Dr K. Kamenarova email: kkamenarova@mmcbg.org Daniela Dacheva, Laboratory manager of MMC email: dacheva@mmcbg.org Chief Asist Prof. A. Mitkova, Project manager of MMC email: mitkova@mmcbg.org Genomics Core Molecular Medicine Centre, Medical University of Sofia 2 Zdrave St., 1431 Sofia, Bulgaria Tel.: (+359) 2 9172 214 http://www.mmcbg.org; mmcbg@abv.bg