ChemE 260 – Thermodynamics
advertisement

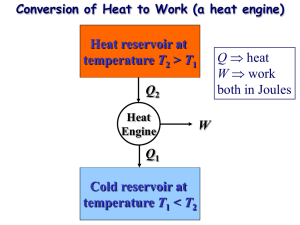
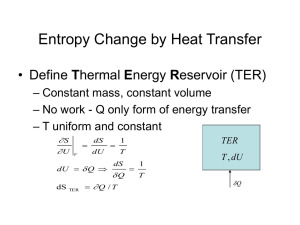
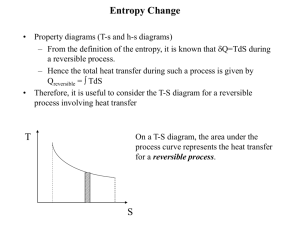
ENGR 224 – Thermodynamics Test #2 – Outline Spring 2011 Chapter 6 – The Second Law of Thermodynamics Introduction to the 2nd Law 1st Law and spontaneity Thermal Reservoirs Thermodynamic Cycles o Heat engines: thermal efficiency o Refrigerators: coefficient of performance o Heat Pumps: coefficient of performance o th , COPR, COPHP = fxns(QH, QC) The 2nd Law o Clausius Statement and Kelvin-Planck Statement o Perpetual motion machines Reversibility and Irreversibility o Sources of irreversibility Friction Rapid compression or expansion Heat transfer through a finite temperature difference Others o Internal and external irreversibility The Carnot Cycle o A reversible heat engine can be reversed to become a refrigerator or heat pump o PV Diagrams for Carnot Cycles o Equipment that can be used to carry out a Carnot Cycle o 1st and 2nd Carnot Principles – latest expression of the 2nd law Thermodynamic Temperature Scales o Kelvin Relationship o Kelvin temperature scale is identical to the ideal gas temperature scale o Carnot (reversible) cycle efficiency and COP = fxns(TH and TC) ONLY. o Plot th , COPR, COPHP as fxns of TH and/or TC. Chapter 7 – Entropy Clausius Inequality o Reversible and irreversible cycles Definition of Entropy o Apply the definition to thermal reservoirs: S = Q / Tres Entropy Data o Thermodynamic tables o TS Diagrams Areas under internally reversible process paths are Q Area enclosed by a Carnot Cycle is W Entropy Generation o Sgen definition and Suniv Qirrev < Area on a TS Diagram The 1st and 2nd Gibbs Equations o Special Cases Solids and incompressible liquids Ideal Gases – Shomate Equation Ideal Gas Entropy Function: So(T) Constant heat capacities Polytropic Processes o Special Cases: Isobaric, isochoric, isothermal, isentropic o Special P, V, T relationships o Wb for polytropic processes o PV and TS Diagrams of polytropic processes o Isentropic Processes Relative properties and how to use them Chapter 8 –Thermodynamics of Flow Processes Entropy Balances on Closed Systems Entropy Balances on Open Systems o Transient and steady-state o SISO and MIMO Mechanical Energy Balance Equation o Internally reversible, steady-state, SISO o Combination of 1st Law the definition of entropy o Special Cases of MEBE Bernoulli – no shaft work ˆ W S Pout Vˆ dP - no shaft work and Epot = Ekin = 0 Pin WS on PV Diagrams WS for polytropic processes Isentropic Efficiency o Turbines, compressors, pumps and nozzles o Accessible States o HS Diagrams Two-Stage Compression with Intercooling o Reduced work to accomplish the same compression o Ideal Gas with Constant Heat Capacities Optimal design: Px Pin Pout and Wb easy to calculate Lost Work o WS,lost WS,rev WS,act o WS,lost Tsurr Sgen This applies for internal, external and total Sgen and WS,lost. Equations were derived for internal, external and total Sgen and WS,lost o Equations for the lost work for cycles were derived Second Law Efficiency o Only defined for processes that produce or consume work o A more reasonable measure of performance than the isentropic efficiency o Ratio of actual and reversible work