SEDIMENTARY ORES
advertisement

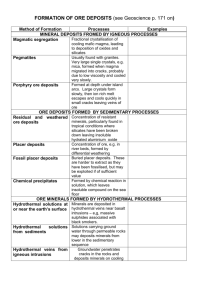
SEDIMENTARY ORES 1. Placer Deposits. These are masses of gravel or sand containing particles of gold (or possibly platinum, tin or other ores).Minerals which develop into placer concentrations must have: (a) high density because……… (b) high resistance to weathering because…………………….. (c) chemical stability because…………………………………… Mark on these diagrams the places where you think placer deposits may form. Why will placers collect in the places you have marked? 2. Residual Deposits. These are formed by chemical weathering which removes soluble materials and leaves insoluble materials which are enriched in metals. E.g. granite weathers in acidic rainwater to form kaolinite (china clay) which was an important mineral found in Cornwall. Extreme chemical of granite leads to the formation of bauxite the main ore of aluminium. Bauxite is earthy and uncemented, so most ores are found in low-lying areas where erosion rates are slow., e.g. Australia and West Africa. Why do bauxites mostly form in tropical climates? Precipitated Deposits. These are formed by deposition from solution in sedimentary environments. Minerals formed by chemical reactions in solution settle out on the sea floor. The most important deposits are banded ironstones, e.g. the Jurassic ironstones in Britain. The main world scale banded ironstones are Pre Cambrian in age and probably formed in anaerobic conditions, possibly aided by bacteria. The conditions of deposition were low energy, shallow water environment. What types of structures in the ironstone deposit show a sedimentary origin? HYDROTHERMAL ORES Warm or hot aqueous solutions moving through rocks can result in hydrothermal ore deposits. The most common hydrothermal ores in Britain are galena, sphalerite and chalcopyrite. Origin of Hydrothermal Solutions The origin is groundwater held in pores and cracks in the rocks. This may have come from Meteoric water Sea water Formation water Magmatic water The deeper in the crust, the higher the temperature of tehwater. Hydrothrmal solutions move through the crust, picking up metal cations . If conditions, e.g. temperature, pressure, pH, change, the metal cations may precipitate rapidly or crystallise slowly to form a mineral deposit. Three places where hydrothermal processes commonly occur are: At or near the crustal surface Deep below the surface in sedimentary basins Deep below the surface near igneous activity.