Momentum and Photons
advertisement

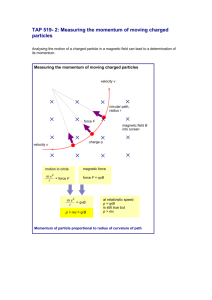
Momentum and Photons Section 12.4 and 12.5 PROBLEM #2: In some experiments, light exhibits particle properties, such as momentum. If an electromagnetic wave interacts with a particle, then the particle oscillates at the same frequency as the wave. However, if the particle absorbs the waves, then the wave cannot be scattered. Scattered x-rays have a different energy than incident x-rays when electrons are ejected from a metal foil. However, if photons are all energy, they shouldn’t experience changes to their energy because they have no mass. Therefore, photons must have mass, meaning… light has particle properties! If the law of conservation of energy is valid, then the energy of the incident x-ray equals the kinetic energy given to the electron plus the energy of the emitted photon. E x ray hf f where 1 mv 2 2 (7) hff is the energy of the emitted x-ray photon ½mv2 is the kinetic energy of the electron that interacted with x-ray Besides energy, momentum is also conserved during collisions. momentum of an object is given by the equation: p mv However, according to Einstein, E = mc2. Therefore, Recall that the m E c2 For a photon, v = c. Therefore, the momentum equation becomes E p c Recall that E hc . Therefore, p where h (8) p is the momentum of the photon (N.s) λ is the wavelength of the photon (m) h is Planck’s constant PROBLEM #3: Electrons, protons, and neutrons exhibit wave characteristics, such as diffraction. It was suggested that since photons have a detectable linear momentum, which is a property of matter, then matter might be explained in terms of waves. Therefore, the momentum equations for photons, h p or h mv were extended to matter, where λ is referred to as the deBroglie wavelength.