Corrected_supporting information
advertisement

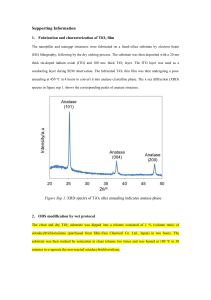
SUPPLEMENTARY MATERIALS Scheme 1. The schematic illustration of ONF-induced visible response of nanostructured TiO2. The ONF generated at the nanostructures can induce the phonon-assisted process, which excites an electron in the valence band of a semiconductor to the conduction band via energy of phonon by the incident photon energy lower than its bandgap Eg. The photocatalytic water splitting performance is evaluated by photoelectrochemical measurement with TiO2 film as working electrode, Ag/AgCl reference and Pt counter electrode. SCHEME 1 1 (1) Fabrication For the fabrication of TiO2 nanorod thin films, the custom-built radio frequency magnetron sputtering system equipped with a glancing angle control system (GLAD) was used. The deposition was performed using the Ti target (50mm in diameter, Thickness 5 mm, and purity 99.99%, purchased from Furuuchi Chemical Corporation) under the mixture of Ar and O2 gases atmosphere. The schematic illustration of GLAD system is shown in the Fig. Sup. 1. TiO2 nanorod film was fabricated onto transparent conducting substrate for the next step of photoelectrochemical (PEC) characterization. During the deposition process the temperature of substrate increase up to 60oC. In order to control the diameters of nanorod, experiments were conducted with different parameters: working distance D, the angle , and the rotation Φ. The morphology and surface energy of the substrate play significant factors for the growth of the nanorods. The mean average diameters of nanorods were estimated from the top-view SEM images by image analysis software Image-J. For the fabrication of nanorod with diameter smaller than 100nm, a flat Indium tin oxide (ITO) coated glass substrates (purchased from Sigma-aldrich, surface 2 resistivity 70-100 Ω/sq) were used. In order to achieve larger nanorods (larger than 100nm), a pyramid-like patterned F-doped tin oxide coated glass substrate (FTO) was used (purchased from Asahi-Glass, surface resistivity 10-15 Ω/sq, Fig. Sup. 2). In this case, the nano-pattern plays as nucleation site for the growth of nanorods. The details about GLAD and gases flow parameters was described in the table Sup. 1. The deposition rate from magnetron flux was controlled by quartz crustal microbalance (QCM) and the thickness of all deposited films was controlled at 300nm for comparative study. The as-deposited films were amorphous TiO2 and after annealed at 550oC under oxygen flow (400 sccm) in 4 hours, they underwent a phase transition from amorphous to the anatase crystal structure. The oxygen flow was used during the annealing process to reduce the oxygen defects in the film. For the preparation of reference flat film, an aqueous solution (50% ethanol; acetic acid 1% added) of 15mg/ml titanium dioxide nanoparticles (Aeroxide P-25 titanium dioxide, NIPPON AEROSIL Co., Ltd.) was prepared. After ultrasonicating in 30 minutes to break up the nanoparticles aggregates, the TiO2 suspension was spin coated onto FTO substrate and dried at 180oC for 2 min. This procedure was repeated 3 into 3 layers in order to obtain the desired thickness (300nm) and finally the whole films were calcinated at 450oC for 2 hours. (2) Structural and optical properties Crystalline structure The films were characterized by X-ray diffraction using Cu-Kα radiation (λ = 0.154 nm) at 40 KV and 300mA with the RINT 2500 instrument. X-ray diffraction (XRD) spectra showed different crystallographic signals from the as-deposited to the 550oC annealed TiO2 nanorod samples. Upon annealing, the XRD spectra of TiO2 films in Fig. Sup. 3(a) show diffraction peaks at 25.27o and 47.96o which corresponds to the anatase phase. The film made of P-25 nanoparticles which are a mixture of anatase and rutile phase at the ratio of 4:1 does show the peak of anatase (101) yet the rutile peaks are very weak, because of the thickness of the film. We also prepared a 10m-thick P-25 film using the same procedure and we confirmed the peaks of rutile with the ratio comparable to its reported crystalline structure Fig. Sup. 3(b). Optical property 4 The UV/vis optical transmission spectra were acquired using the Spectrophotometer UV-Visible-NIR Hitachi U-4100 instrument. The optical bandgap of fabricated TiO2 thin films were determined using the following relationship, for TiO2 is an indirect semiconductor from the Tauc plot: hconst hg; where his photon energy, is absorption coefficient at wavelength λ; g is bandgap energy of material. The optical bandgap of 65nm nanorod, 150nm nanorod and P-25 flat film were calculated from transmittance and reflectance spectra to be 3.06eV, 3.25eV and 3.22eV respectively, which corresponds well to bulk anatase TiO2. The transmittance spectra were used to calculate the absorbance of films. (3) Photoelectrochemical measurement Photoelectrochemical (PEC) measurement was performed in a custom-built 3-electrode cell (Pt wire counter electrode, Ag/AgCl reference electrode, and TiO2 thin film working electrode) with an equipped quartz window of 15mm in diameter. For UV irradiation, the light source Ushio SP-9 Spot Cure equipped with a 365nm band-pass filter was used. For visible irradiation, the 488nm Argon ion laser was used and the irradiation spot diameter was changed in the range of 1mm ~ 15mm to vary the power density. The power was calibrated and measured using the USHIO - Unimeter UIT-250 5 power meter and ADCMT 8230E optical power meter for UV (365nm) and visible (488nm) irradiation, respectively. Prior to PEC experiments, all solutions were deaerated with argon gas. All the power dependence measurements under UV and visible irradiation were acquired at 0.47V (vs SCE, or 0.5V vs Ag/AgCl) external bias voltage. The photocurrent was acquired by the ALS electrochemical analyzer Model 814B. The typical I-t curves under irradiation were described in Fig. Sup. 4. The initial decrease in the photocurrent may be attributed to the increase in the recombination rate with the increased trapping of the charge carriers during illumination, since the mass transfer limitation of the hydroxide ions in the hole scavenging process is negligible. The same tendency has been observed for both flat film and nanorod samples. The photocurrent density was calculated from the I-t curves from 6s after the light-on as the I-t curves reach the steady state. (4) Photoluminescence (PL) measurement The PL spectra were acquired at 12K using the SPEX1702 system. The films were excited by Hd-Cd laser (325nm), with the excitation power density estimated to be 6 10Wcm-2 (excitation spot diameter equal to 0.2mm). The PL signals were detected using the photon counting photomultiplier R1387 with the slit width of 2mm. The film thickness is lower than the penetration of the excitation beam, there is a slight of PL signals at ~380nm which is derived from the substrate. The spectra after subtracting signals derived from the substrates were shown in the Figure Sup. 5. The spectra were then normalized with the absorbance and fitted by Gaussian fitting using GRAMS/AI software developed by Thermo Scientific Corp. 7 FIGURE CAPTIONS FOR SUPPORTING INFORMATION Figure Sup. 1. The schematic of magnetron sputtering system equipped with GLAD regime Figure Sup. 2. The top view SEM image of pyramid-like patterned F-doped Tin Oxide coated glass Figure Sup. 3. (a) XRD spectra of 300nm-thick TiO2 films (b)The XRD spectra of 10m-thick thin film made of Aeroxide P-25 nanoparticles. The peak at 2 = 27.56 o can be ascribed to rutile phase, and the ratio between anatase and rutile is comparable to the reported crystal structure of P-25 nanoparticles Figure Sup. 4. Typical time-resolved photocurrent I-t curves under (a) UV 365nm and (b) visible 488nm irradiation Figure Sup. 5. The PL spectra of TiO2 films after subtracting the background 8 FIGURE SUP. 1 9 FIGURE SUP. 2 10 FIGURE SUP. 3 11 FIGURE SUP. 4 12 FIGURE SUP. 5 13