PBS Learning Media Simulation: Tutorial Covalent Bonding http
advertisement

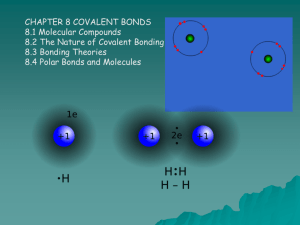
PBS Learning Media Simulation: Tutorial Covalent Bonding http://www.pbslearningmedia.org/resource/lsps07.sci.phys.matter.covalentbond/ covalent-bonding/ This interactive activity from ChemThink describes covalent bonding—a type of chemical bond that involves the sharing of electrons. Investigate the attractive and repulsive forces that act on atomic particles and how the sharing of electrons can keep atoms together. See how two hydrogen atoms interact with each other to create a covalent bond. Learn about trends in the periodic table and how electrostatic potential energy determines the bond length. Also, learn about naming conventions for covalent compounds. Follow the instructions closely as you move through this activity! There are some screens where you have to do something before you can move onto the following screen. This media asset was adapted from ChemThink. Student Questions 1. In the two hydrogen atoms simulation, how is the movement of electrons different when the atoms are close? Explain the electron location using electric force and Coulomb’s Law. What happens if you try to move the atoms too close to each other? 2. What is a covalent bond? 3. What type of elements form covalent bonds? Why does hydrogen behave differently from other elements in its group when it comes to bonding? 4. In the potential energy simulation, why does the potential energy between the atoms decrease as you move them closer together? 5. In the potential energy simulation, after a certain point, the potential energy increases if you try to move the atoms any closer. Why does this happen? 6. What determines the bond length of a covalent bond? 7. In terms of stability, why do atoms form covalent bonds? 8. In the formula H-H, what does the line represent? 9. How many pairs of electrons does oxygen share? Depict oxygen bonding using lines to represent bonds. 10. How many electrons total are shared in a triple bond? 11. Triple bonds are strongest, followed by double bonds and single bonds are weakest. Explain this trend using electric force and Coulomb’s Law in your answer. 12. Write the names of the following covalent compounds: a. b. c. d. e. f. g. h. i. j. k. N2O NO2 N2O4 N2O3 NO H2O S2O SO3 S2O3 SO2 S2Cl2