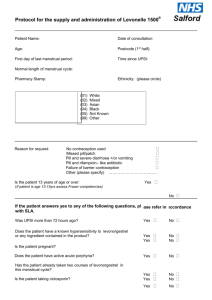
PATIENT GROUP DIRECTION FOR THE SUPPLY OF HORMONAL POST
COITAL CONTRACEPTION – LEVONORGESTREL 1,500 mcg tablets
(LEVONELLE 1500) TO PATIENTS BY COMMUNITY PHARMACISTS
You must be authorised by name, under the current version of this PGD
before you attempt to work according to it.
Validity
From:
Valid until:
Review date:
Clinical Condition
Indication
To prevent pregnancy after unprotected sexual intercourse
(UPSI) or failure of contraceptive method.
Inclusion Criteria
Exclusion Criteria
Patients who are at risk of pregnancy owing to sexual
intercourse having taken place without protection or
where protection has failed and
presenting within 72 hours of unprotected sexual
intercourse
12 -16 years who fulfil the Fraser Guidelines and
over 16
Cautions/Need
for further advice
Under 12 years of age
Suspected pregnancy
Patients with previous untreated episode/s of
unprotected sexual intercourse in current menstrual
cycle
Overdue menstrual bleed due to unknown cause
(i.e. NOT due to breast feeding, recent use of
depot, no pill free break etc)
Patients presenting later than 72 hours
Active / Acute / Severe Liver disease
Allergy to levonorgestrel or a constituent of the
product
Porphyria
Patients who are currently taking a liver enzyme inducing
drug below should take TWO doses at once (See Dose
Section). This is an unlicensed use.
Phenobarbitone, phenytoin, primidone, carbamazepine,
oxcarbazepine, topiramate, griseofulvin, rifampicin,
PGD for the supply of levonorgestrel 1500mcg (Levonelle 1500) by Community Pharmacists
Page 1 of 8
rifabutin, amprenavir, atazanavir, nelfinavir, saquinavir,
ritonavir, efavirenz, nevirapine, lansoprazole, tacromlimus,
tretinoin, bosentan, modafinil, aprepitant, St Johns Wort,
fluconazole, itraconazole, ketoconazole, terbinafine
Check concurrent medication with the client for liver
enzyme inducing drugs and other interacting drugs using
BNF
Action if patient
declines or is
excluded
Drug Details
Name, form &
Strength of
medicine
Route/Method
Refer to Medical Practitioner, Family Planning Services /
Contraception and Sexual Health Services or out of hours
provider e.g. Central Nottinghamshire Clinical Services
(CNCS) and Nottingham Emergency Medical Service
(NEMS)
Document all exclusion criteria and reason for not
supplying EHC
Levonorgestrel 1500 micrograms (Levonelle 1500)
1 tablet
Oral
Legal Category
Prescription Only Medicine (POM)
Dosage
Levonorgestrel 1500 micrograms (1 tablet) as soon as
possible, preferably within 12 hours, and no later than 72
hours after unprotected intercourse
If the patient is taking a liver enzyme inducing drug (refer
to cautions section), the dose should be 3000 micrograms
at once. This is an unlicensed use. This advice is not
contained within the Summary of Product Characteristics
(SPC), but follows the Faculty of Family Planning and
Reproductive Health Care (FFPRHC) recommendations
Frequency
Single dose of 1 tablet (except patients taking liver enzyme
inducing drugs)
A further dose of 1 tablet can be taken if vomiting occurs
within 2 hours of taking the first tablet
Duration or
minimum
treatment period
Although Levonelle 1500 is licensed for a single course
per menstrual cycle, a second dose may be given to
patients presenting for a second dose within one
menstrual cycle (if PGD inclusion and exclusion criteria are
still met) but the pharmacist should consult clinician where
possible and advise patient that this should NOT be
regarded as regular method of contraception. Advice
PGD for the supply of levonorgestrel 1500mcg (Levonelle 1500) by Community Pharmacists
Page 2 of 8
about regular and future contraception should also be
given. Advice should be recorded.
Quantity to
1 tablet (2 tablets for patients taking liver enzyme inducing
supply/administer drugs)
Concurrent
Medication
Side effects/
Adverse
outcomes
Check concurrent medication with the patient or carer for
interactions using BNF. Not to be given to patients taking
medication previously highlighted under exclusion criteria.
Nausea is the most common side effect
Vomiting (If vomiting occurs within 2 hours of taking
the dose the patient should be advised to return for
another dose as soon as possible)
Headache, dizziness, fatigue, low abdominal pain
and breast tenderness
Disruption of the normal pattern of menstrual
bleeding
If pregnancy does occur after taking Levonelle
1500, the possibility of an ectopic pregnancy should
be considered. The absolute risk of ectopic
pregnancy is low. While there is no evidence of any
risk of teratogenicity with Levonelle 1500, a normal
outcome to any pregnancy cannot be guaranteed.
Cautions for use – The rates of efficacy are 95% if
taken within 24 hours of unprotected sexual
intercourse, 85% 24-48 hours and 58% if started
between 48-72 hours.
Please refer to BNF or SPC for further information on sideeffects, cautions etc.
Patients or carers should be advised to report adverse
outcomes to the appropriate healthcare professional (e.g.
patient’s GP). Nottinghamshire County Teaching PCT
incident reporting form must be completed and procedure
guidelines followed.
All serious outcomes should be reported using the Yellow
Card System to report adverse drug reactions directly to
the MHRA www.yellowcard.gov.uk and as per local
procedures. Yellow Cards and directions and guidance on
its use are available at the back of the BNF.
Advice to patient
Method of administration
Possible side effects
Small possibility of failure of treatment
If vomiting occurs within 2 hours of taking the dose,
the patient should return for another dose
All methods of post coital contraception should be
discussed, including Intrauterine device (IUD)
PGD for the supply of levonorgestrel 1500mcg (Levonelle 1500) by Community Pharmacists
Page 3 of 8
Follow up
Treatment and
referral
Concurrent use of broad spectrum antibiotics –
Levonelle 1500 is not affected by broad spectrum
antibiotics – therefore, no special action is needed
Advise appointment at family planning clinic or GP
as soon as possible if next period is missed,
delayed by MORE than 5 days or lighter than usual
to exclude pregnancy
Advise on need for reliable contraception until the
end of this cycle as one dose protects for 1 incident
only
Discuss future contraception
Discuss risk of Sexually Transmitted Infections /
HIV
Levonorgestrel 1500 micrograms may be given to
breast feeding women. Potential exposure to an
infant to levonorgestrel can be reduced if breast
feeding women take the dose immediately after
feeding.
Severe malabsorption syndromes e.g. Crohns
disease may impair the efficacy of levonorgestrel.
Advise appointment at family planning clinic or GP
as soon as possible.
If client is on warfarin, advise client that additional
INR monitoring may be required
If client is on ciclosporin, advise client that
levonorgesterel may inhibit the metabolism of
ciclosporin by the liver and thus increase blood level
of ciclosporin. Advise the client to report to the GP
if they experience an increase in side effects
If client is under 16, an assessment on Fraser ruling
must be made and documented
Routine follow up is not required but in cases of
uncertainty relating to the supply of EHC under this PGD,
the pharmacist should contact or refer the client to her GP
or Family Planning Clinic
Staff Characteristics
Qualifications
Pharmacists currently registered with the Royal
Pharmaceutical Society of Great Britain
Specialist
Competencies or
Qualifications
1) Has undertaken recent training (within the last 3 years)
of the CPPE pack Emergency Hormonal Contraception
(ref 36456)
OR
Has undertaken training of the CPPE pack Emergency
Hormonal Contraception (ref 36456) and has been
providing the service on a regular basis within the last 3
years
PGD for the supply of levonorgestrel 1500mcg (Levonelle 1500) by Community Pharmacists
Page 4 of 8
2) Has completed the training sessions on supply of EHC
under PGD (including supply to the under 16s)
organised by one of the following organisations –
Ashfield and Mansfield District PCTs / Bassetlaw PCT
/ Southern Derbyshire PCT / Nottingham City PCT /
Gedling PCT / Rushcliffe PCT / Broxtowe and Hucknall
PCT before April 2008 and has been providing the
service on a regular basis within the last 3 years
3) For pharmacists that have met criteria 1 and 2, they
have up to XXXXXXXXXX to complete a conversion
course or attend a conversion course developed by
Nottinghamshire County Teaching PCT
4) For new pharmacists (pharmacists that do not meet
criteria 1 and 2), they need to complete the CPPE
training on PGD (ref 38034) Child Protection (ref
37025) and Emergency Hormonal Contraception (ref
36456) AND attend a training event organised by
Nottinghamshire County Teaching PCT or successfully
complete a competency assessment developed by
Nottinghamshire County Teaching PCT.
Facilities &
Supplies
Levonelle should be obtained by pharmacists from a bona
fide supplier and should be stored in the pharmacy at room
temperature (not exceeding 25C)
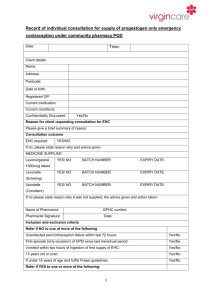
Records and
Audit Trail
Management and
monitoring
Client Consultation record form (Signed by client and
pharmacist)
Fraser assessment form - for clients under 16
Pharmacy computer records
Monitoring will be undertaken during routine pharmacy
reviews/monitoring
Audit of Client Consultation record forms will be
undertaken by the Primary Care Trust.
PGD for the supply of levonorgestrel 1500mcg (Levonelle 1500) by Community Pharmacists
Page 5 of 8
References:
Levonelle 1500 Summary of Product Characteristics October 2005
Faculty of Family Planning and Reproductive Health Care Statement on
Levonelle 1500 and use of liver enzyme inducing drugs April 2006
Faculty of Family Planning and Reproductive Health Care Guidelines for
Emergency Contraception April 2004 revised June 2003
Faculty of Family Planning and Reproductive Health Care. Clinical Effectiveness
Unit Drug Interactions with Hormonal Contraception 2005; 31 (2): 139.151
British National Formulary 55 (March 2008)
PGD for the supply of levonorgestrel 1500mcg (Levonelle 1500) by Community Pharmacists
Page 6 of 8
PATIENT GROUP DIRECTION FOR THE SUPPLY OF HORMONAL POST
COITAL CONTRACEPTION – LEVONELLE 1500 TO PATIENTS BY
COMMUNITY PHARMACISTS
This Patient Group Direction has been developed by:
Signature…………………………………..
Date……………………
Jacqueline Liew, Pharmacy Liaison Advisor, Nottinghamshire County
Teaching PCT
Signature…………………………………..
Date……………………
Dr Simone Reuter, Associate Specialist, Family Planning Services,
Nottinghamshire County Teaching PCT
Signature…………………………………..
Date……………………
Jan Kochanowski, Nurse Team Leader, Family Planning Services,
Nottinghamshire County Teaching PCT
This Patient Group Direction has been authorised for use by:
Signature…………………………………..
Date……………………
Amanda Sullivan, Director of Nursing and Integrated Governance,
Nottinghamshire County Teaching PCT
Signature…………………………………..
Date……………………
Doug Black, Medical Director, Nottinghamshire County Teaching PCT
PGD for the supply of levonorgestrel 1500mcg (Levonelle 1500) by Community Pharmacists
Page 7 of 8
PATIENT GROUP DIRECTION FOR THE SUPPLY OF HORMONAL POST
COITAL CONTRACEPTION – LEVONELLE 1500 TO PATIENTS BY
COMMUNITY PHARMACISTS
Please note that
It is the responsibility of each professional to practice only within the
bounds of their own competence and in accordance with their own Code
of Professional Conduct
Patient Group Directions do not remove inherent professional obligations
or accountability
I have received, read and understood
This Patient Group Direction
The Fraser guidelines for assessing competence in clients under 16 years
of age
I agree to act as an approved pharmacist and to supply and/or administer this
medicine only in accordance with this PGD.
I have received the training which approved pharmacists MUST undertake before
authorisation to supply emergency hormonal contraception under this PGD
Name of approved
pharmacist
Designation
Signature
Date
Name and address of Pharmacy
PGD for the supply of levonorgestrel 1500mcg (Levonelle 1500) by Community Pharmacists
Page 8 of 8