MifeprexAlternativeTxCI - TEACH | Training in Early Abortion for
advertisement

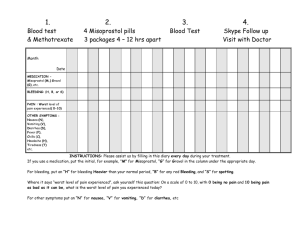
PLANNED PARENTHOOD GOLDEN GATE EASTMONT, HAYWARD, MACARTHUR, REDWOOD CITY, ROHNERT PARK, SAN FRANCISCO, SAN MATEO & SAN RAFAEL To get information or to make an appointment at any of the above health centers please call: 1-800-967-PLAN (7526) Client Information and Consent for Alternative Treatment Plans for Mifeprex (mifepristone) The Mifeprex Medication Guide and Patient Agreement provided by the supplier describe the treatment plan for Mifeprex currently approved by the Food and Drug Administration (FDA). This plan is: Medications: 600 mg dose of Mifeprex (mifepristone) by mouth and 400 mcg of misoprostol by mouth. Mifeprex taken at the clinic on Day 1. Misoprostol taken at the clinic on Day 3. Follow up in the clinic on Day 13-16. Use of the medications up to 49 days of pregnancy. In research studies, other treatment plans for mifepristone have been shown to be equally safe and effective for medical abortion. We offer other options for using Mifeprex including: A. Medication Dosing Changes. 1. A lower dose of 200 mg of Mifeprex (one pill) can be used instead of the 600 mg (three pills) dose recommended on the package. 2. When the 200 mg dose of Mifeprex is used, the dosage and method of using the second drug misoprostol, must be changed. The dose of misoprostol is then 800 mcg (four pills) and they are inserted high into the vagina instead of taken orally. There have been several clinical research studies with the 200 mg oral dose of Mifeprex and the 800 mcg vaginal dose of misoprostol. In some studies, this combination of 200 mg of Mifeprex (mifepristone) by mouth and 800 mcg of misoprostol vaginally has been shown to: Cause less nausea and vomiting than the dosage combination in the current package labeling. Be equally effective at ending early pregnancy. Be acceptable to women. B. Change in the day misoprostol is taken and expanding the length of pregnancy medical abortion can be used. Option 1 If a woman is 63 days pregnant or less she can take 200 mg of Mifeprex (mifepristone) by mouth and on Day 2 or 3 take 800 mcg misoprostol vaginally. Length of pregnancy will be measured by ultrasound. Research has shown that mifepristone can be safely and effectively used up to 63 days of pregnancy when using the 200 mg dose of mifepristone taken by mouth and the 800 mcg dose of misoprostol inserted into the vagina on Day 2 or 3 of the procedure. Option 2 If a woman is 56 days pregnant or less she can take 200 mg of Mifeprex (mifepristone) by mouth and on Day 2, 3 or 4 takes 800 mcg misoprostol vaginally. Length of pregnancy will be measured by ultrasound. Research has shown that mifepristone can be safely and effectively used up to 56 days of pregnancy when using the 200 mg dose of mifepristone taken by mouth and the 800 mcg dose of misoprostol inserted into the vagina on Day 2, 3, or 4 of the procedure. However, in these studies women who were CO6 04/02 PATIENT INFO AND CONSENT FOR ALTERNATIVE TREATMENT PLANS FOR MIFEPREX Page 1 of 3 asked to take their misoprostol on Day 4 often did not wait till Day 4 to take their misoprostol because they wanted the abortion procedure to be finished earlier. C. Choosing to take the second medication (misoprostol) at home instead of in the clinic. Studies have shown that women can take the second drug (misoprostol) themselves without a health care provider being present. Therefore, taking misoprostol at home is safe, effective and acceptable to women. Most women abort within 2 to 24 hours, (average is 4 hours) after taking the second drug, misoprostol. Women in studies preferred to take their misoprostol at home because of privacy, convenience, control, and comfort, among other reasons. D. Option to omit the office visit on the day misoprostol is taken with a telephone call instead. It is safe and effective to take misoprostol at home when misoprostol is taken, without an in-person visit to the doctor’s office or clinic. In research studies women were given packages of misoprostol at their first visit (Day 1). They were instructed to take their dose of misoprostol at home on Day 2, 3, or 4. No extra visit to the clinic or doctor’s office to pick up the misoprostol was needed on Day 2, 3, or 4. In research studies most women preferred not to have a clinic or doctor’s office visit to pick up or take their misoprostol. Women preferred picking up the misoprostol on Day 1. Most reported that the bleeding and cramping following misoprostol were managed at home more comfortably and conveniently. To review the information on page 1: The FDA-approved treatment plan in the Mifeprex package is: The latest day for the abortion is 49 days since the last period. The medication is 600 mg dose of Mifeprex (mifepristone) by mouth and 400 mcg of misoprostol by mouth. The second visit is an in-person visit to the clinic on Day 3. Misoprostol is taken in the clinic on Day 3. The follow up appointment is in the clinic on Day 13-16. Please read the following agreement and check your choices below: I request the following alternative treatment plan for my medical abortion. Alternative Treatment Plans Please check and initial your choices below _____ I am 63 days pregnant or less and my medication will be 200 mg dose of Mifeprex by mouth on Day 1. I will then take 800 mcg (4 tabs) of misoprostol vaginally on Day 2 or Day 3 according to how I have been instructed. _____ I am 56 days pregnant or less and my medication will be 200 mg dose of Mifeprex by mouth on Day 1. I will then take 800 mcg (4 tabs) of misoprostol vaginally on Day 2, 3 or 4 of the procedure according to how I have been instructed. _____ I will take the misoprostol at home myself. ____ On Day 1, I will be given my misoprostol to take home, with instructions on which day to take it at home myself. I will arrange with Planned Parenthood for the telephone call on or around the day I take my misoprostol. I can be reached by telephone during the day at:__________________________________ CO6 04/02 PATIENT INFO AND CONSENT FOR ALTERNATIVE TREATMENT PLANS FOR MIFEPREX Page 2 of 3 ______I do not choose any of the above alternatives. I choose to receive Mifeprex according to the Patient Agreement and Medication Guide in the Mifeprex package. I have been given information about different methods of taking Mifeprex and misoprostol to end my pregnancy. I have read and understood the treatment plan described in the Medication Guide and Patient Agreement provided in the Mifeprex (mifepristone) packaging that was approved by the FDA. I understand the alternative options for taking Mifeprex (mifepristone) using other treatment plans. These methods have been studied extensively and found to be as safe and effective as the method approved by the FDA. My provider has answered all of my questions about the different methods of taking Mifeprex and misoprostol. Based on this information, I have chosen the method that is the best choice for me. I have indicated that choice by the boxes I have checked above. I understand that my choice may be a change from the method approved by the FDA as stated in the Patient Agreement required by the FDA. By signing below, I consent to the treatment plan I have checked and initialed above. If I have chosen an alternative treatment plan, I hereby modify the Patient Agreement. Signature of Patient: __________________________________________Date:_________________ I witnessed the fact that the patient received the above-mentioned information and said she/he read and understood same and had the opportunity to ask questions. Signature of Witness: _________________________________________Date:_________________ CHECK HERE IF PATIENT’S GUARDIAN OR RELATIVE IS LEGALLY REQUIRED TO SIGN BELOW Signature of any other person consenting _____________________________________________ Relationship to patient ______________________________________Date___________________ I witnessed the fact that the patient’s legal guardian (or person consenting in her behalf) received the above-mentioned information and said she read and understood same. Signature of Witness _____________________________________________Date_____________ CO6 04/02 PATIENT INFO AND CONSENT FOR ALTERNATIVE TREATMENT PLANS FOR MIFEPREX Page 3 of 3