Example
advertisement

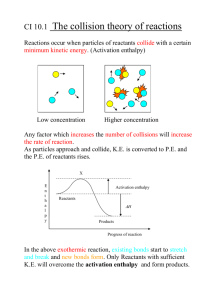
ThermoChemistry Summary Definitions: a) enthalpy – heat given off or absorbed at constant pressure. b) exothermic – energy, in the form of heat given off to surroundings. c) First Law of Thermodynamics – energy can neither be created nor destroyed. d) system – the small part of the Universe we are interested in – in this case the chemicals in the reaction e) calorimetry – measuring the heat of a process. f) standard enthalpy of formation – heat associated with the formation of one mole of a compound from its elements in their standard state (way they occur at 1 atm and 25 oC). g) endothermic – energy in the form of heat, absorbed (taken in) by the chemicals (system) from the surroundings. The standard enthalpy of formation or standard heat of formation of a compound is the change of enthalpy that accompanies the formation of 1 mole of the compound from its elements, with all substances in their standard states. Its symbol is ΔHfO or ΔfHO. The superscript theta (zero) on this symbol indicates that the process has been carried out under standard conditions. Standard States are as follows: 1. For a gas: standard state is a pressure of exactly 1 bar 2. For a substance present in a solution: a concentration of exactly 1 M at a pressure of 1 bar 3. For a pure substance in a condensed state (a liquid or a solid): the pure liquid or solid under a pressure of 1 bar 4. For an element: the form in which the element is most stable under 1 bar of pressure and the specified temperature. (Usually 25 degrees Celsius or 298.15 K) One exception is phosphorus: most stable under 1 bar is black phosphorus, but white phosphorus is used as the reference for zero enthalpy of formation [1] For example, the standard enthalpy of formation of carbon dioxide would be the enthalpy of the following reaction under the conditions above: C(s,graphite) + O2(g) → CO2(g) Note that all elements are written in their standard states, and one mole of product is formed. This is true for all enthalpies of formation. The standard enthalpy of formation is measured in units of energy per amount of substance. Most are defined in kilojoules per mole (kJ mol−1), but can also be measured in calories per mole, joules per mole or kilocalories per gram (any combination of these units conforming to the energy per mass or amount guideline). In physics the energy per particle is often expressed in electronvolts which corresponds to about 100 kJ mol−1. All elements in their standard states (oxygen gas, solid carbon in the form of graphite, etc.) have a standard enthalpy of formation of zero, as there is no change involved in their formation Standard enthalpy of reaction The standard enthalpy of formation is used in thermochemistry to find the standard enthalpy change of reaction. This is done by subtracting the sum of the standard enthalpies of formation of the reactants (each being multiplied by its respective stoichiometric coefficient, ν) from the sum of the standard enthalpies of formation of the products (each also multiplied by its respective stoichiometric coefficient), as shown in the equation below: ΔH° = Σ(ν × ΔHf°) (products) - Σ(ν × ΔHf°) (reactants) ALWAYS: ∆Hrxn is PRODUCTS - REACTANTS EXAMPLE: for the reaction CH4 + 2 O2 → CO2 + 2 H2O: ΔHr° = [(1 × ΔHf°(CO2)) + (2 × ΔHf°(H2O))] (products) - [(1 × ΔHf°(CH4)) + (2 × ΔHf°(O2))] (reactants) If the standard enthalpy of the products is less than the standard enthalpy of the reactants, the standard enthalpy of reaction will be negative. This implies that the reaction is exothermic. The converse is also true; the standard enthalpy of reaction will be positive for an endothermic reaction. Reading Potential Energy Graphs: In endothermic reactions, heat energy is taken in from the surroundings, and turned into potential energy in the products. As a result, the enthalpy of the products is greater than the enthalpy of the reactants. The enthalpy change for an endothermic reaction is always positive. Example The potential energy (enthalpy) diagram for an endothermic reaction is shown left: The activation energy (Ea) for the forward reaction is shown by (A): Ea (forward) = H (activated complex) - H (reactants) = 400 - 100 = 300 kJ mol-1 The activation energy (Ea) for the reverse reaction is shown by (B): Ea (reverse) = H (activated complex) - H (products) = 400 - 300 = 100 kJ mol-1 The enthalpy change for the forward reaction is shown by (C): H = H (products) - H (reactants) = 300 - 100 = 200 kJ mol-1 Reactants Ep is higher than Products Ep. ► Now, we must consider the activation energy (the energy needed so that the reactants bonds will break and reform to make product) ► This is EXOTHERMIC. Heat exits the chemicals in order to have energy content in the products. ► ∆H will be NEGATIVE lower ► Reactants Ep is lower than Products Ep. ► Need to add more energy to the system for the forward reaction to take place. ► This is ENDOTHERMIC. Heat (energy) must be added to the system (chemicals) to raise the energy content of the products higher than that of the reactants. ► ∆H will be POSITIVE ► Still need to consider activation energy Fast & Slow Reactions ► The smaller the activation energy, the faster the reaction will occur regardless if exothermic or endothermic. ► If there is a large activation energy needed, that means that more energy (and therefore, time) is being used up for the successful collisions to take place. ► Catalyst: a substance that helps the reaction go faster, but is not used up in the reaction. A catalyst usually reduces the activation energy, thus making the reaction go faster.