Topic(s) The Chemical Basis for Life: Carbon Compounds
advertisement

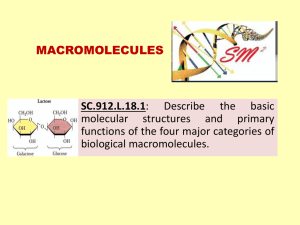
1. 2. 3. 4. 5. Topic(s) The Chemical Basis for Life: Carbon Compounds, Carbohydrates and Lipids Curriculum Map Location October Essential Questions What are the building blocks and functions of carbohydrates, lipids, proteins, and nucleic acids? Why do living things need food? Key Concepts Carbon can bond with many elements, including hydrogen, oxygen, phosphorus, sulfur, and nitrogen to form the molecules of life. Living things use carbohydrates as their main source of energy. Plants, some animals, and other organisms also use carbohydrates for structural purposes. Lipids can be used to store energy. Some lipids are important parts of biological membranes and waterproof coverings. Some proteins control the rate of reactions and regulate cell processes. Some proteins build tissues such as bone and muscle. Others transport materials or help fight disease. Nucleic acids store and transmit hereditary, or genetic, information. Anchor Descriptor** BIO.A.2.2 Describe and interpret relationships between structure and function at various levels of biochemical organization (i.e., atoms, molecules, and macromolecules). Eligible Content** BIO.A.2.2.1 Explain how carbon is uniquely suited to form biological macromolecules. BIO.A.2.2.2 Describe how biological macromolecules form from monomers. BIO.A.2.2.3 Compare the structure and function of carbohydrates, lipids, proteins, and nucleic acids in organisms. Performance Assessment (Formative Assessment—on going) 1. 2. Exit Tickets Informal Observation (structured pair-work): teacher monitors and makes note addressing common misunderstandings and gaps in understanding. Through cues or prompts redirect gaps or misunderstandings 3. Make an Appointment: group members share with each other and that group will then go to a different to compare responses. 4. Thumbs Up and Thumbs Down: Pair opposite or split group based on arguments which would discuss concepts further 5. Develop Series of Questions: students share thinking with teacher or members of group, students learn if he or she has full, partial, or no understanding of the concept 6. Students analyze responses to open-ended question(s) made from other student responses—results in reflect on own learning 7. Group projects enabling a number of students to work together on a complex problem that requires planning, research, internal discussion, and group presentation. 8. Essays/Short Answers assessing students' understanding of a subject through a written description, analysis, explanation, or summary. 9. Experiments testing how well students understand scientific concepts and can carry out scientific processes. 10. Demonstrations giving students opportunities to show their mastery of subject-area content and procedures. 11. Portfolios/Binders allowing students to provide a broad portrait of their performance through files that contain collections of students' work, assembled over time #1-5 Most commonly used on a day to day basis MUST KNOW --- KEYSTONE VOCABULARY FOR THESE TOPICS 1) 2) 3) 4) Adenosine Triphosphate (ATP) -A molecule that provides energy for cellular reactions and processes. ATP releases energy when one of its high‐energy bonds is broken to release a phosphate group. Biological Macromolecules - A group of biomacromolecules that interact with biological systems and their environments. Carbohydrate -A macromolecule that contains atoms of carbon, hydrogen, and oxygen in a 1:2:1 ratio and serves as a major source of energy for living organisms (e.g., sugars, starches, and cellulose). Catalyst -A substance that enables a chemical reaction to proceed at a usually faster rate or under different conditions (e.g., lower 1 5) 6) 7) 8) 9) 10) 11) 12) 13) temperature) than otherwise possible without being changed by the reaction. Deoxyribonucleic Acid (DNA) -A biological macromolecule that encodes the genetic information for living organisms and is capable of self‐replication and the synthesis of ribonucleic acid (RNA). Enzyme - A protein that increases the rate of a chemical reaction without being changed by the reaction; an organic catalyst. Lipids -A group of organic compounds composed mostly of carbon and hydrogen including a proportionately smaller amount of oxygen; are insoluble in water, serve as a source of stored energy, and are a component of cell membranes. Macromolecule -A polymer with a high molecular mass. Within organisms there are four main groups: carbohydrates, lipids, proteins, and nucleic acids. Molecule -The smallest particle of a substance that retains the chemical and physical properties of the substance and is composed of two or more atoms held together by chemical forces. Monomer -A molecule of any compound that can react with other molecules of the same or different compound to form a polymer. Each biological macromolecule has characteristic monomers. Nucleic Acid - A biological macromolecule (DNA or RNA) composed of the elements C, H, N, O, and P that carries genetic information. Organic Molecule - A molecule containing carbon that is a part of or produced by living systems. Protein -A macromolecule that contains the principal components of organisms: carbon, hydrogen, oxygen, and nitrogen; performs a variety of structural and regulatory functions for cells. Learning Target (student friendly) I can or will…. Start Date: Nov 16th explain why carbon is unique and how the carbon atom bonds to form a variety of compounds. Completion Date: Nov 19th the differences between inorganic and organic compounds and define Resources Monomers and Polymers Macromolecules use a cut-and-tape paper exercise to help me define monomer and polymer and show how monomers can form polymers as well as explain the difference between dehydration synthesis (AKA condensation reaction) and hydrolysis Tell you what purpose carbohydrates serve. Tell what the simplest form of a carbohydrate is and provide 3 examples Explain how 3 monosacharides: glucose, galactose, fructose are similar and different Tell what a disaccharide is, how it is formed (by use of paper cut-outs), and an example Tell what a polysaccharide is, how it is formed (by use of paper cut-outs), and an example name 3 types of polysaccharide and where they are stored (2 plant and 1 animal) and each purpose tell you what a lipid is, its purpose, and why it is not soluble in water tell what monomer most lipids are made of and describe its Announcement/Comments: Lesson plans subject to change according to students need. Students will be required to maintain a binder (portfolio) which is an organized presentation of your child’s work and skills throughout the year. 2 structure describe the structure of a triglyceride tell you difference between good (unsaturated) fats and bad (saturated) fats and where they are found tell you what phospholipid is and its purpose describe the structure of waxes and steroids, provide examples, and their purpose REVIEW the overall concepts of Properties of Water. Carbon Compounds, Carbohydrates and Lipids 1. 2. Labs/Demonstrations/Activities/Tests (Monday through Friday in numerical order) Lab 4 Exercise Organic Molecules: Lipids Review for Test on Properties of Water, Carbon Compounds, Carbohydrates, & Lipids 4. Test Corrections on PWCCL—Friday Nov 20th 3. Test on Properties of Water, Carbon Compounds, Carbohydrates, & Lipids---Thursday Nov 19th !!! ** Anchors and Eligible Content is the foundation upon what ASD Biology curriculum was developed and therefore connects to the biology curriculum map 3