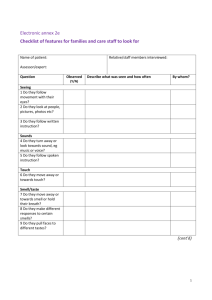
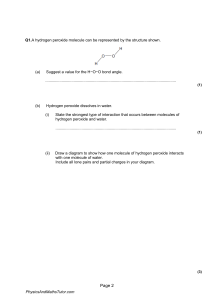
Experiment O03 Chemical properties of ethanol
advertisement

Experiment O03 Chemical properties of ethanol Results Table (Reactions of ethanol) Property/Reaction A B Observations a. Solubility in water a. Miscible. b. pH of solution. b. PH = 6 a. Pungent smell. Mild oxidation: a. Smell. b. Fehling's solution. c. Silver mirror test. b. Colour changes from blue to red. c. A silver mirror developed on inside of the tube. C Further oxidation: a. Smell. b. Universal indicator paper. c. Sodium carbonate. a. Vinegary smell. b. pH = 3 c. Effervescence. D Triiodomethane reaction Pale yellow ppt formed. E Reaction with sodium Gas evolved popped with lighted splint. Evaporation gave white solid. F Esterification Pleasant, sweet smell. G Dehydration a. Decolorized. a. Bromine water b. Acidified potassium permanganate b. Decolorized. solution Questions 1. Which of the reactions of ethanol produced a reducing agent? What is the reducing agent? Reaction B. Ethanal. 2. Which of the reactions of ethanol produced an acidic compound? The smell should give you a clue as to what it might be. What is the acidic compound? Reaction C. Ethanoic acid. 3. What conditions and relative proportions of reactants are used in the oxidation of ethanol to favour the production of (a) ethanal, (b) ethanoic acid? (a) Ethanal: 1. Lower temperature, 2. Ethanol in excess. (b) Ethanoic acid: 1. Higher temperature, P.8 2. Oxidizing agent in excess.