Human Papilloma virus, Genotyping
advertisement

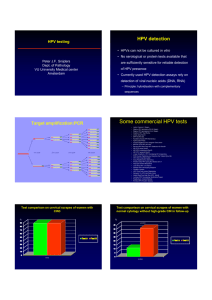
Technical Laboratory Bulletin Topic: Human Papilloma virus, Genotyping Test Name Test Code Method Changes Specimen Test Schedule Human Papilloma virus, Genotyping HPVGE Signal Amplification, Cervista IVD test Revised Method 2011 ThinPrep vial, cervical/non-cervical sources Thursdays Change: HPV genotyping will be performed using the Cervista HPV 16/18 IVD test. HPV genotyping can be ordered on patients that have tested positive for the HPV high risk screen (HPVHR, HPVH, or HPVHO). Indications: Human papilloma viruses are a group of more than 100 different viruses, many of which have been shown to cause cancer. Several "high risk" types of HPV are associated not only with cervical cancer, but also with various other cancers including anal, penile, and throat cancer. HPV16 and HPV18 are the most prevalent genotypes in squamous cell carcinoma and in adenocarcinomas The FDA-approved clinical indications for the Cervista HPV 16/18 test include: Women over the age of 30 in conjunction with cervical cytology and a positive HPV high-risk screen. Women with ASC-US cervical cytology in conjunction with a positive HPV high-risk screen. NOTE: A negative result for the HPV 16/18 genotyping test does not exclude the presence of other high risk HPV subtypes. Correlation with clinical findings and other laboratory data is warranted. Method: DNA is extracted and the presence of HPV types 16 and 18 are evaluated via signal amplification using the Cervista HPV 16/18 IVD test. This assay detects only the high risk HPV genotypes 16 and 18. Result Reporting: Positive or Negative for the high risk HPV genotypes16 and/or 18. References: 1. 2. 3. 4. American Society for Colposcopy and Cervical Pathology, HPV Genotyping Clinical Update, 2009 Cervista HPV 16/18 [package insert]. Madison, WI: Third Wave Technologies, Inc; 2009 G. D’Souza, et al. Case–Control Study of Human Papillomavirus and Oropharyngeal Cancer N Engl J Med 2007;356:1944-56. Chris J. Meijer a, Peter J. Snijders a, Philip E. Castle b,⁎ Clinical utility of HPV genotypingGynecologic Oncology 103 (2006) 12–17 Additional Information: Contact Hayley Webber, Ph.D., Molecular Technical Director or Monica Ianosi-Irimie, M.D.,Ph.D., Laboratory Director, NorDx, at (207) 396-7800, or Email webbeh@mmc.org