CARBOHYDRATES
advertisement

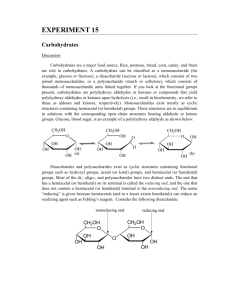
Name: _________________________________ Per:________ Performance: ________ BIOCHEMISTRY LAB # 6 CARBOHYDRATES BACKGROUND Carbohydrates are polyhydroxy compounds that are either aldehydes or ketones that contain only carbon, hydrogen, and oxygen. Carbohydrates comprise about 60% of our daily caloric intake and are the major source of metabolic energy. Carbohydrates are categorized as monosaccharides, disaccharides, or polysaccharides. Monosaccharides: Are the simplest carbohydrates and serve as the building blocks for di and poly saccharides. Glucose, galactose, and fructose are examples of monosaccharides. All digestible dietary carbohydrates in our diet are eventually broken down into glucose, and therefore it is also commonly known as blood sugar. Foods that tend to elevate blood sugar levels too high and too fast are thought to contribute to insulin resistance and type 2 diabetes. Fructose is found in fruits and fruit juices and in honey. Fructose is also commonly known as fruit sugar, and can be found in a large number of foods items (as high fructose corn syrup). All monosaccharides are reducing sugars (i,e, they can be oxidized), and either contain an aldehyde (aldoses) or a ketone (ketoses) group. Disaccharides: In a disaccharide two monosaccharides are linked together via a glycosidic bond. Maltose, lactose, and sucrose are three common disaccharides. Lactose, also commonly known as milk sugar, is found in milk and milk products. Lactose contains a glucose and galactose combined via a glycosidic bond. Individuals who lack the enzyme to breakdown (digest) lactose suffer from lactose intolerance. Sucrose, common table sugar, is composed of glucose and fructose. Unlike the monosaccharides, not all disaccharides are reducing sugars. Of the three common disaccharides Lactose and Maltose are reducing sugars, while Sucrose is not. Polysaccharides: Polysaccharides are polymers composed of multiple monosaccharide units. Amylose, Amylopectin, Glycogen, and Cellulose are all polysaccharides containing glucose, and are different only in the manner in which glucose units are attached to each other. Starch composed of the polysaccharides amylose and amylopectin, is the major form of glucose storage in plants. Amylose (~ 20% of starch) is a linear chain of glucose while amylopectin (~ 80%) is a branched polymer of glucose. Glycogen is the major form of glucose storage in animals, and is found in the liver and muscle. Glycogen, like amylopectin in plants, is a branched polymer of glucose. The breakdown (hydrolysis) of glycogen helps maintain blood glucose levels between meals. Cellulose is an unbranched chain of glucose and is the major structural component of plants. Humans do not have the enzymes to digest cellulose and therefore, humans cannot digest cellulose. Such undigestable carbohydrates provide the major source of fiber in our diet. Tests for the identification of carbohydrates Benedict’s test for reducing sugars: Benedict’s reagent reacts with reducing sugars to form a red precipitate. The color of the precipitate can vary from green to gold to red depending on the amount (concentration) of the reducing sugar present in the sample. Seliwanoff’s test for ketoses: Seliwanoff’s test can be used to distinguish 6 carbon monosaccharides (hexoses) that have a ketone group from those hexoses that contain an aldehyde group. With ketoses the reagent produces a deep red color rapidly, while with aldoses a light pink color develops over a longer period of time. Name: _________________________________ Per:________ Performance: ________ Iodine test for polysaccharides: Iodine interacts with the structure of amylose to produce a deep blueblack complex. The polysaccharides amylopectin, glycogen, and cellulose, interact with iodine to give red to brown colors. Glycogen gives a reddish-purple color with iodine. Mono and di- saccharides do not interact with iodine to form dark colored complexes. HYDROLYSIS OF DISACCHARIDES AND POLYSACCHARIDES Disaccharides and polysaccharides can be hydrolyzed (broken down), given sufficient time, into their constituent monosaccharides. In the laboratory hydrolysis can be achieved by reacting the di- and polysaccharides with acid. Acid hydrolysis of disaccharides will produce the constituent monosaccharides. Acid hydrolysis of polysaccharides will first produce smaller polysaccharides and disaccharides, but eventually, given sufficient time, will produce the constituent monosaccharides. In our bodies the hydrolysis of polysaccharides (except cellulose) is carried out by enzymes that are found in our saliva or secreted from our pancreas. PROCEDURE The goal of this laboratory is to introduce you to the concepts related to carbohydrates. You will first carry out the three identification reactions on samples containing known carbohydrates and record your observations. Based on these observations you will then attempt to identify the carbohydrate contained in unknown solutions. Second, you will hydrolyze a carbohydrate using acid. Once the carbohydrate has been treated with acid you will test for hydrolysis of the sample using the Benedict’s test and the iodine test. You will compare the samples with acid to samples that did not contain any acid to determine if the carbohydrate was hydrolyzed. Finally you will test a common food item for the presence of different carbohydrates using the three tests you performed in the first part. Materials: One large beaker (250-400 mL), plastic well plate, micro-centrifuge tubes and clips, boiling water bath, floating test tube rack, hot plate, beral pipettes, and plastic 24-well plate. A. BENEDICT’S TEST FOR REDUCING SUGARS Materials: Benedict’s reagent and 2% carbohydrate solutions: glucose, fructose, sucrose, lactose, 1% starch and unknown. Place 5-6 drops of solutions of glucose, fructose, sucrose, lactose, starch, water and an unknown in separate, labeled micro-centrifuge tubes. Add 9 drops of Benedict’s reagent to each sample, then cap and gently shake the closed tube to mix. Heat all the tubes in a boiling water bath for 3-4 minutes. The formation of a greenish to reddish-orange color indicates the presence of a reducing sugar. If the solution is the same color as the Benedict’s reagent in water (the control), then there is no oxidation reaction. Record your observations. Classify each as reducing or non-reducing sugar. B. Seliwanoff’s Test for Ketoses Materials: Seliwanoff’s reagent and 2% carbohydrate solutions: glucose, fructose, sucrose, lactose, 1% starch and unknown. Place 2-3 drops of solutions of glucose, fructose, sucrose, lactose, starch, water and unknown in separate, labeled micro-centrifuge tubes. Add 10 drops of Seliwanoff’s reagent to each sample, then cap and gently shake the closed tube to mix. The reagent contains concentrated HCl. Use carefully. Name: _________________________________ Per:________ Performance: ________ Place the test tubes in a boiling hot water bath and note the time. After 5 minutes, observe the colors in the tube. The formation of a deep red color indicates the presence of a ketose. Record your results as a deep red color change, pale red/pink change or no change. C. IODINE TEST FOR POLYSACCHARIDES Materials: Iodine reagent and 2% carbohydrate solutions: glucose, fructose, sucrose, lactose, 1% starch and unknown. Place 5-6 drops of each solution of glucose, fructose, sucrose, lactose, starch, water and an unknown in separate wells in your well plate. Add 1 drop of iodine reagent to each. A dark blue-black color is a positive test for amylose in starch. Record your results. Complete the table to identify the carbohydrates in your unknown. D. HYDROLYSIS OF DISACCHARIDES AND POLYSACCHARIDES Materials: 10% HCl, 10% NaOH, red litmus paper, iodine reagent, Benedict’s reagent, 1% starch and 2% sucrose solutions, stir rod. Place 7-8 drops of 1% starch in two micro-centrifuge tubes and 7-8 drops of sucrose in two more microcentrifuge tubes. To one sample of sucrose and starch, add 3-4 drops of 10% HCl. To the other samples of sucrose and starch, add 3-4 drops of DI water. Label the test tubes and gently shake the closed tubes to mix, and then heat in a boiling water bath for 10 minutes. Remove the test tubes from the water bath and let them cool. To the samples containing HCl, add 10% NaOH (about 3-4 drops) and shake the closed tube to mix. Add NaOH until the solution turns red litmus paper blue, indicating the HCl has been neutralized. Apply a drop of solution from the tip of the stir rod onto the litmus paper to check pH. Test the samples for hydrolysis as follows: Iodine Test: Place 5-6 drops of each solution in a well and add 1 drop of iodine solution to each. Add extra iodine solution if the color disappears. Continue to add iodine solution one drop at a time until a permanent color is obtained. Record your observations. Determine if hydrolysis has occurred in each. Benedict’s Test: Add 9 drops of Benedict’s reagent to each of the remaining samples and heat in a boiling water bath for 3-4 minutes. Determine if hydrolysis has occurred in each. E. TESTING FOODS FOR CARBOHYDRATES Materials: Obtain a carbohydrate sample to test. Perform Benedict’s, Seliwanoff’s and iodine tests on each. Describe the kinds of carbohydrates you identify in each sample. PRE-LABORATORY QUESTIONS- 2PTS EACH Read the lab procedures, then answer the pre-lab questions on a separate sheet of paper. Points will be taken off for sloppy work… 1. Draw the reaction of glucose with the Benedict’s reagent. 2. Draw the hydrolysis reaction of sucrose, structures and names. 3. Based on your knowledge about the different tests for Carbohydrates, predict the outcome (final color) of the Benedicts test and the Seliwanoff’s test with Sucrose, before and after hydrolysis. 4. List three examples each for monosaccharides, disaccharides and polysaccharides. For the di- and poly- saccharides also list its constituent monosaccharide. 5. Write the formula for sucrose, maltose and lactose REPORT SHEET A. BENEDICT’S, B. SELIWANOFF’S AND C. IODINE TESTS FOR CARBOHYDRATES Compound Observations A. Benedict’s Glucose Fructose Sucrose Lactose Starch Water Unknown #_________ B. Seliwanoff’s C. Iodine D. HYDROLYSIS OF DISACCHARIDES AND POLYSACCHARIDES Test Observations Sucrose + H2O Sucrose + HCl Starch + H2O Starch + HCl Iodine Benedict’s E. TESTING FOODS FOR CARBOHYDRATES Food sample tested: _______________________________________ Test Observations Benedict’s Seliwanoff’s Iodine 5 QUESTIONS 1. In parts A, B, and C you performed three tests to identify various carbohydrates. Based on your observations for the known solutions and your observations for the unknown solution, what carbohydrate(s) is/are in your unknown solution? Provide a brief explanation as to how you made your choice. 2. Which sucrose and starch samples in part D undergo hydrolysis? How do the observations for the iodine and Benedict’s tests indicate that hydrolysis has occurred? 3. Based on your observations in part D what are the hydrolysis products for each tube: Sucrose + H2O Sucrose + HCl Starch + H2O Starch + HCl 4. Based on your observations in part E what possible carbohydrates are present in the food item you tested? 5. You have in front of you three vials containing a clear liquid. You are told that each vial contains one of the following carbohydrates: Fructose, Sucrose, Amylose. What experiments could you carry out to determine the composition of each vial. Write down the experiment(s) as well as the expected observations. 6