Mucosal immune cell numbers and visceral sensitivity in
advertisement

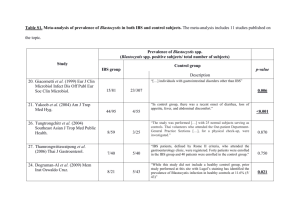
The relation between stress, microscopic inflammation and visceral sensitivity in patients with irritable bowel syndrome. B. Braak,1, *, T. K. Klooker1, *, M. M. Wouters2, O. Welting3, C. M. van der Loos4, O. I. Stanisor3, S. van Diest 3, R. M. van den Wijngaard3 and G. E. E. Boeckxstaens1,2,5. 1 Department of Gastroenterology and Hepatology, AMC, Amsterdam, the Netherlands; 2Translational Research Center for Gastrointestinal Disorders, University Hospital Leuven, Catholic University Leuven, Leuven, Belgium; 3Tytgat Institute of Liver and Intestinal Research, AMC, Amsterdam, the Netherlands; 4Department of Pathology, AMC, Amsterdam, the Netherlands, 5Department of Gastroenterology, University Hospital Leuven, Catholic University Leuven, Leuven, Belgium. *Both authors participated equally and sharing first authorship. ABSTRACT Repeated exposure to stress leads to mast cell degranulation, microscopic inflammation and subsequent visceral hypersensitivity in animal models. To what extent this pathophysiological pathway plays a role in patients with the irritable bowel syndrome (IBS) has not been properly investigated. The aim of this study was to assess the relationship between visceral hypersensitivity, microscopic inflammation and the stress response in IBS. Microscopic inflammation of the descending colonic mucosa was evaluated by immunohistochemistry in IBS patients and healthy volunteers (HV). Rectal sensitivity was assessed by a barostat study using an intermittent pressure-controlled distension protocol. Salivary cortisol to a series of 3 psychological stress tests (i.e. stroop test (color-word conflicting test), mirror tracing test and public speech test) was measured to assess the stress response in a subgroup of patients. peak cortisol levels, expressed as the maximal increase from baseline, was calculated (median (range), ug/ml). 66 IBS patients (74% female (F), age 38 ± 2 yr) and 20 HV (65% F, 31 ± 3 yr) were included in this study. Of all IBS patients, 52% (n=33) were considered visceral hypersensitive to rectal distension (i.e. threshold of discomfort <24 mm Hg above MDP). In IBS, mast cells (IBS: 186 ± 10 vs. HV: 370 ± 39 / mm2, p<0.001) CD8 Tcells (IBS: 388 ± 28 vs. HV: 526 ± 50/ mm2, p=0.02) and macrophages (IBS: 729 ± 64 vs. HV: 1261 ± 146/ mm2, p<0.003) were decreased . Similarly, free light chain (FLC) positive mast cells were decreased (IBS: 1 (0-34) vs. HV: 7 (0-33) /mm2, p=0.004), but not IgE- and IgG positive mast cells. There were no differences between hypersensitive and normosensitive IBS patients. No relation was found between any of the immune cells studied and the thresholds of discomfort, urge or IBS symptoms. 22 IBS and 18 HV underwent the stress test. Baseline and peak cortisol were comparable between IBS and HV (IBS: baseline 5.3 (22.1), peak 1.3 (18.3); HV: baseline 6.8 (19.7), peak 1.3 (29.6); NS). The HPA-axis response to stress was not correlated with the number of mast cells (IBS: r=0.41, p=0.1; HV: r=0.26, p=0.3) or the presence of visceral hypersensitivity. CONCLUSIONS: Our data show a reduction of the number of mast cells, macrophages, T-cells and FLC positive mast cells in IBS compared to HV. There is no association between the number of these immune cells and the presence of visceral hypersensitivity or abnormal stress response. Our data question the role of microscopic inflammation as underlying mechanism of visceral hypersensitivity, but rather suggest dysregulation of the mucosal immune system in IBS.