Single Cell Biosensor coupled to Capillary Electrophoresis
advertisement
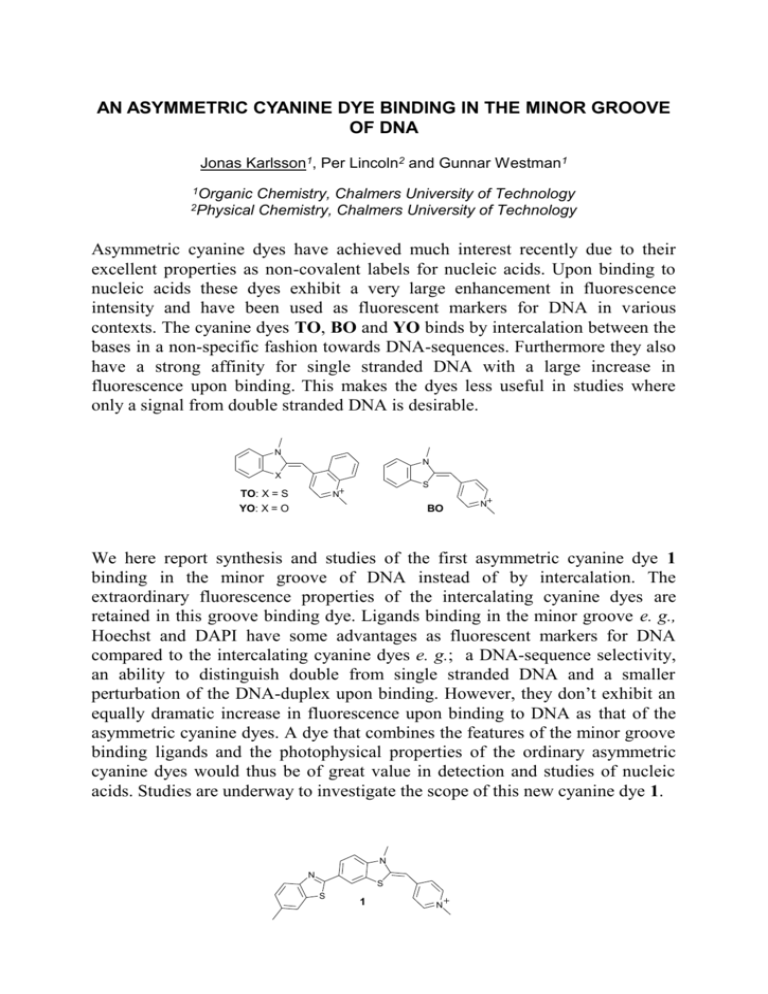
AN ASYMMETRIC CYANINE DYE BINDING IN THE MINOR GROOVE OF DNA Jonas Karlsson1, Per Lincoln2 and Gunnar Westman1 1Organic 2Physical Chemistry, Chalmers University of Technology Chemistry, Chalmers University of Technology Asymmetric cyanine dyes have achieved much interest recently due to their excellent properties as non-covalent labels for nucleic acids. Upon binding to nucleic acids these dyes exhibit a very large enhancement in fluorescence intensity and have been used as fluorescent markers for DNA in various contexts. The cyanine dyes TO, BO and YO binds by intercalation between the bases in a non-specific fashion towards DNA-sequences. Furthermore they also have a strong affinity for single stranded DNA with a large increase in fluorescence upon binding. This makes the dyes less useful in studies where only a signal from double stranded DNA is desirable. N N X S TO: X = S N YO: X = O BO N We here report synthesis and studies of the first asymmetric cyanine dye 1 binding in the minor groove of DNA instead of by intercalation. The extraordinary fluorescence properties of the intercalating cyanine dyes are retained in this groove binding dye. Ligands binding in the minor groove e. g., Hoechst and DAPI have some advantages as fluorescent markers for DNA compared to the intercalating cyanine dyes e. g.; a DNA-sequence selectivity, an ability to distinguish double from single stranded DNA and a smaller perturbation of the DNA-duplex upon binding. However, they don’t exhibit an equally dramatic increase in fluorescence upon binding to DNA as that of the asymmetric cyanine dyes. A dye that combines the features of the minor groove binding ligands and the photophysical properties of the ordinary asymmetric cyanine dyes would thus be of great value in detection and studies of nucleic acids. Studies are underway to investigate the scope of this new cyanine dye 1. N N S S 1 N






