Seagrass_Literature_Review - Department of Environment

Seagrass and Reef Program for Port Phillip
Bay: Seagrass Literature Review
F. Y. Warry and J. S. Hindell
2009
Arthur Rylah Institute for Environmental Research
Arthur Rylah Institute for Environmental Research
Review of Victorian seagrass research, with emphasis on Port Phillip Bay.
F. Y. Warry and J. S. Hindell
Arthur Rylah Institute for Environmental Research
123 Brown Street, Heidelberg, Victoria 3084
November 2009
Arthur Rylah Institute for Environmental Research
Department of Sustainability and Environment
Heidelberg, Victoria
Report produced by: Arthur Rylah Institute for Environmental Research
Department of Sustainability and Environment
PO Box 137
Heidelberg, Victoria 3084
Phone (03) 9450 8600
Website: www.dse.vic.gov.au/ari
© State of Victoria, Department of Sustainability and Environment 2009
This publication is copyright. Apart from fair dealing for the purposes of private study, research, criticism or review as permitted under the Copyright Act 1968 , no part may be reproduced, copied, transmitted in any form or by any means
(electronic, mechanical or graphic) without the prior written permission of the State of Victoria, Department of
Sustainability and Environment. All requests and enquiries should be directed to the Customer Service Centre, 136 186 or email customer.service@dse.vic.gov.au
Citation: Warry, F. Y. and Hindell, J. S. (2009) Review of Victorian seagrass research, with emphasis on Port Phillip
Bay. Arthur Rylah Institute for Environmental Research. Draft Report. Department of Sustainability and Environment,
Heidelberg, Victoria
ISSN 1835-3827 (print)
ISSN 1835-3835 (online)
ISBN XXXXXXXX (print)
ISBN XXXXXXXX (online)
Disclaimer: This publication may be of assistance to you but the State of Victoria and its employees do not guarantee that the publication is without flaw of any kind or is wholly appropriate for your particular purposes and therefore disclaims all liability for any error, loss or other consequence which may arise from you relying on any information in this publication.
Front cover photo: Heterozostera nigricaulis at Grassy Point, Port Phillip Bay (P. I. Macreadie).
Authorised by: Victorian Government, Melbourne ii
Contents
iii
iv
List of figures
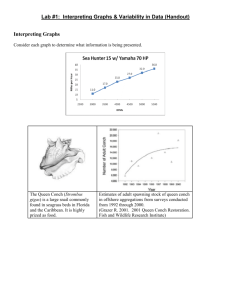
Figure 1: Conceptual model depicting size, depth and hydrodynamic energy relationships among
(ian.umces.edu/symbols/), University of Maryland Center for Environmental Science. ....... 24
(ian.umces.edu/symbols/), University of Maryland Center for Environmental Science. ....... 25
v
Acknowledgements
Earlier versions of this review were improved by comments from P. Reich. vi
Review of Victorian Seagrass Research
Summary
Victorian seagrass research was reviewed to synthesise current understanding of seagrass ecosystems, with particular emphasis on Port Phillip Bay. Specifically, this review covers the biology and ecology of seagrass, identifies threats, and options for assessing seagrass condition and addressing decline.
Zostera mulleri and Heterozostera spp. have been the focus of most seagrass research in Victoria.
Amphibolis antarctica has received recent attention as a result of the Channel Deepening Project in
Port Phillip Bay. Halophila australis, Posidonia australis and Lepilaena marina have been the subject of limited research in Victoria.
Seagrass distribution and structure varies across spatial and temporal scales and among species.
Victorian seagrass distributions have typically been mapped using ground-truthed aerial photography. The distributions of different species overlap to some degree, but many occur in different parts of the environment. Historical comparisons indicate a pattern of frequent, localised and small-scale fluctuations across Port Phillip Bay. Periods of broad-scale decline have been observed in Western Port and Corner Inlet during the last fifty years. Seagrass structure influences faunal assemblages. The relationship between physical structure and the resilience and function of seagrass ecosystems is not completely understood in Victoria.
Biological factors and environmental conditions influence seagrass distribution and physical structure. Reproductive strategies and growth rates influence recruitment and recovery following disturbance events. The role of sexual reproduction versus clonal growth in sustaining seagrass ecosystems is presently unclear. The role of genetic diversity in promoting seagrass resilience is also poorly understood. Critical patch sizes and levels of structural complexity affecting the ability of seagrasses to modify their environment have not been quantified in Victoria.
Understanding mechanisms and rates of recruitment and patch-growth is crucial in assessing recovery of seagrass.
The influence of environmental factors, including hydrodynamics, sediment, nutrients, light and water quality on seagrass distribution and structure has been addressed to varying degrees.
Interactions between and among biological and environmental factors are likely to be important but are poorly understood.
Victorian seagrasses support productive and diverse algal and faunal communities; these communities exhibit spatio-temporal variability. Assessments of seagrass communities in Victoria have focused on Z. mulleri and Heterozostera spp. Some work has focused on A. antarctica and P. australis but comprehensive assessments of H. australis and L. marina communities are lacking.
Seagrasses support juvenile life-stages of macrofauna (e.g. fish) but their importance as nursery habitat remains equivocal, largely because the contributions of juveniles from seagrass habitats to adult populations have not been quantified.
Seagrasses perform a range of ecosystem functions. Catchment-based and landscape scale phenomena influence the way seagrass systems function. Catchment-derived nutrients have been assimilated by seagrasses in Victorian bays and inlets and incorporated into food webs. Nutrient enrichment affects the growth and condition of Z. mulleri and Heterozostera spp.
and will likely have implications for other Victorian species, but these have not been studied. Long term implications of nutrient enrichment on seagrass condition are unclear. Landscape-scale configurations and processes, such as habitat fragmentation, affect patterns of faunal assemblages
(e.g. density, abundance, species richness) in Victorian seagrass habitats, but effects on functional processes, including nutrient cycling and trophic transfers are unclear. Seagrasses support
Victorian estuarine and marine food webs. Trophic subsidies to other habitats and the relative
Arthur Rylah Institute for Environmental Research 1
Review of Victorian Seagrass Research contribution of different seagrass species to food webs are unclear. Seagrasses are thought to play an important role in carbon cycles, carbon dioxide sequestration and carbon storage, although these functions are poorly understood.
Major threats to Victorian seagrass systems stem from human population pressures, and climate change. Interactions among threats and impacts on seagrass condition are complex and not fully understood. Assessment of seagrass condition in Victoria has focused on physical attributes of seagrass which makes it difficult to detect changes prior to seagrass decline and measurements of seagrass physiological condition may be better early warning indicators of stress. Transplantation of seedlings, seeding, and recruitment enhancement techniques have been developed elsewhere to restore and rehabilitate seagrass following loss or decline. These techniques may be applicable in
Victoria, but managing threats to seagrasses to promote seagrass resilience and prevent loss will ultimately be the most successful and cost-effective approach to long-term management.
2 Arthur Rylah Institute for Environmental Research
Review of Victorian Seagrass Research
1 Introduction
1.1
Background
Seagrasses are marine angiosperms that occupy shallow nearshore environments. Seagrasses provide important ecological, social and commercial functions. They act as ‘ecosystem engineers’, stabilising sediments and attenuating water flow, and as bio-indicators or ‘coastal canaries’. Seagrasses support high biodiversity and provide habitat for species of recreational and commercial value. Seagrasses also perform important ecosystem functions, including trophic subsidies and carbon storage.
Several species of seagrass occur in Victorian waters. Those considered in this review include:
Amphibolis antarctica
Halophila australis
Heterozostera nigricaulis
Heterozostera tasmanica
Posidonia australis
Zostera mulleri
The water mat Lepilaena marina , which is equivocally classified as a seagrass also occurs in sheltered Victorian waters and is listed as threatened under the Flora and Fauna Guarantee Act
(1988). Until recently, H. nigricaulis and H. tasmanica were considered a single species ( H . tasmanica , see Kuo 2005) Both species have been recorded from Victoria, however, only H. nigricaulis has been confirmed growing in Port Phillip Bay (Kuo 2005). Due to the recent revision of the genus, H. nigricaulis and H. tasmanica will be referred to as Heterozostera in the present review. These two Heterozostera taxa are morphologically very similar and may function in ecologically similar ways. These species are also grouped together in current seagrass maps
(Blake & Ball 2001a, b, Ball et al. 2006).
Seagrasses occur throughout Victorian coastal waters, but are most common in bays and inlets.
The distributions of particular species are influenced by biological and environmental parameters.
Several species occur throughout the state (e.g. Heterozostera, Z. mulleri ), while others have more restricted distributions (e.g. P. australis is largely confined to Corner Inlet, see section 2.1).
This review provides a synthesis of current understanding of Victorian seagrass systems, with particular focus on Port Phillip Bay. It is not an attempt to detail an exhaustive collection of empirical studies. Victorian-focused seagrass research provides the basis for the review, but studies from elsewhere have been used to improve understanding or provide context.
1.2
Approach taken in this review
Information on the form, function, condition and management of seagrass ecosystems was gathered from the primary literature, technical reports and publications and the authors’ knowledge. The review exclusively focuses on seagrass studies, except where work from other habitats was considered relevant. The review largely focuses on A. antarctica, Heterozostera and
Z. mulleri, as these species have been the focus of most Victorian seagrass research.
This review will emphasise areas of research that have provided important information on seagrass ecosystems in Victoria. Biological and environmental factors influencing seagrass distribution
(section 2) and those processes influencing seagrass ecosystem function (section 4) will be emphasised. The nature of seagrass communities (section 3, which have been surveyed extensively in Victoria, particularly Port Phillip Bay) and management issues (section 5) will be covered briefly.
Arthur Rylah Institute for Environmental Research 3
Review of Victorian Seagrass Research
2 Seagrass plants and populations
2.1
Seagrass distribution in Victoria
The predominant species of seagrass growing in Victorian nearshore systems include Amphibolis antarctica, Halophila australis, Heterozostera nigricaulis, Heterozostera tasmanica, Posidonia australis and Zostera mulleri . Lepilaena marina (listed as threatened under the Flora and Fauna
Guarantee Act 1988) also occurs in Victorian waters.
Seagrasses typically occur in shallow coastal waters (< 15 m depth), exposed to low-moderate hydrodynamic energy (see section 2.4.1), predominately within bays and inlets in Victoria. A. antarctica, H. australis, H. nigricaulis, and Z. mulleri occur throughout the state (Roob & Ball
1997, Blake et al. 2000, Blake & Ball 2001a, b, Ierodiaconou & Laurenson 2002). H. tasmanica only occurs in certain locations, from Portland to Wilson’s Promontory, and has not been collected in situ from Port Phillip Bay (Kuo 2005). Extensive beds of P. australis only occur within Corner
Inlet. L. marina has only been recorded from sheltered locations, such as Swan Bay (DCE 1991 as cited in Blake and Ball 2001a).
In Victoria, different species of seagrass often overlap to some degree, but many occur in different parts of the environment. The general patterns of seagrass species distributions with depth and habitat types are best characterised in Corner Inlet, where:
Z. mulleri mainly occupies intertidal areas
Heterozostera typically occurs subtidally to a depth of up to 3-4 meters (depending on local turbidity and incident light levels)
P. australis
H. australis inhabits shallow, subtidal waters occurs in deeper (> 2-3 m depth) waters, often associated with soft, fine sediments.
It often grows in association with Heterozostera at the deeper margins of Heterozostera distribution (Roob et al. 1998)
A. antarctica grows in coarse sediments in subtidal waters exposed to moderate hydrodynamic energy, and forms vast meadows close to the entrance of Port Phillip Bay. L. marina is most common in shallow protected waters, and commonly occurs in similar types of environments to
Zostera .
The most comprehensive Victorian seagrass maps are based on ground-truthed, aerial photography. Areas of seagrass are digitised from photographs using GIS applications, and seagrass species and density attributes are incorporated from field data (Blake et al. 2000, Blake &
Ball 2001a, b, Ball et al. 2006). Z. mulleri and Heterozostera have often been grouped for mapping purposes because they are difficult to differentiate from aerial photographs.
In Port Phillip Bay, Zostera/Heterozostera is the dominant seagrass, accounting for ca. 60 km 2 (95
%) of the seagrass (Blake & Ball 2001a). The most extensive areas occur in Swan Bay, Corio Bay and the Geelong Arm, particularly along the southern shoreline. Large patches also run parallel to the shore of the Bellarine Peninsula, around Mud Island, and at Rosebud, Blairgowrie and
Sorrento. The extent of seagrass is minimal on the eastern shore of Port Phillip Bay (Blake & Ball
2001a). H. australis and A. antarctica account for 2 % and 3 %, respectively, of the area of seagrass in Port Phillip Bay. H. australis is restricted to deeper areas of Swan Bay, the Geelong
Arm and Corio Bay in association with Heterozostera . A. antarctica dominates the Port Phillip
Heads region, typically growing in the vicinity of rocky reefs and interspersed among macroalgae.
A. antarctica has not been recorded east of Point King or north of Queenscliff (Blake & Ball
2001a).
4 Arthur Rylah Institute for Environmental Research
Review of Victorian Seagrass Research
The overall distribution of seagrass within Port Phillip Bay has remained relatively constant since
1957 (Blake & Ball 2001a). Quantitative assessments indicate a pattern of frequent, localised, small-scale fluctuations in the areal extent of seagrasses (Blake & Ball 2001a). The direction of such fluctuations can vary among sites (Ball et al. 2006, Hirst et al. 2009d) and with seasons.
Seagrass density, areal extent and maximum depth have been shown to decline in Port Phillip Bay during autumn/winter due to lower temperatures and shorter photoperiods (Hirst et al. 2009c, Hirst et al. 2009d).
The accuracy of seagrass habitat mapping based on aerial photographs depends on the degree to which digitised habitat classifications accurately represent what is actually there (and can be ground-truthed). In the 2000 seagrass map for Port Phillip Bay (Blake & Ball 2001a), 81 % of the total area mapped was ground-truthed (Blake & Ball 2001a) providing confidence in the map.
Seagrass mapping conducted between 2005 – 2007 at selected sites within Port Phillip Bay,
Western Port and Corner Inlet have combined spatial extent with measurements of ‘condition’, including epiphytic cover, blade length, shoot and flower density, seed densities in the sediment and shoot and rhizome biomass (Ball et al. 2006). Incorporating these variables into seagrass maps improves assessments of spatial and temporal variation in seagrass condition.
Hyperspectral remote sensing techniques are useful for remotely mapping the spatial distributions of different species of seagrass, as well as biomass and foliage composition, e.g. chlorophyll content. Individual spectral reflectance signatures have been detected for three seagrass species in
NSW, although there can be substantial spatial and temporal intraspecific variation (Fyfe 2003).
Intraspecific variation may be useful in the early detection of perturbations. The potential of hyperspectral techniques in remotely detecting changes in species distributions and mapping features beyond areal extent has been demonstrated in other parts of Australia, but these techniques have not been employed in Victoria.
Predictive models, for example those based on classification and regression trees, can generate predicted species distribution maps based on a suite of environmental variables, such as depth and wave exposure (Fonseca et al. 2002). Decision tree classification analysis in conjunction with multibeam echosounder and underwater video ground-truthed data have been used to produce biogenic habitat models and maps in western Victoria (Ierodiaconou et al. 2007, Rattray et al.
2009). Such approaches will be useful for mapping seagrasses (Ierodiaconou et al. 2007), particularly in deeper waters where conventional mapping approaches based on aerial photography are not appropriate. Predicative models may be useful in identifying areas suitable for restoration or rehabilitation (Fonseca et al. 2002). Comparison of predictive models with known seagrass distributions may also help to detect perturbations by identifying areas that appear suitable for seagrasses yet have not been colonised. The current activities of Future Coasts in mapping and modelling inundation regimes along Victoria’s coastline will provide valuable information on how distributions of seagrass may change with increasing sea levels.
2.2
Seagrass structure
The structural complexity of vegetated habitats influences the composition and function of faunal assemblages (see section 3, Jenkins & Sutherland 1997). Key parameters contributing to seagrass patch structure include substratum cover, above- and below-ground biomass, leaf height, leaf and shoot density and plant architecture (Bulthuis 1983a, Ball et al. 2006). Structure varies among seagrass species. The degree of epiphytic coverage on seagrass leaves (see section 3) also influences the structural complexity of seagrass habitats.
Arthur Rylah Institute for Environmental Research 5
Review of Victorian Seagrass Research
Seagrass structure varies spatially at multiple scales. Structure can vary within seagrass patches, and among patches and locations. Above-ground biomass, leaf density and height of
Heterozostera vary from patch edges to interiors in Port Phillip Bay, and are typically greater in the middle of patches (Smith et al. 2008). Structural attributes of seagrasses in Port Phillip Bay also vary substantially among patches and locations (Jenkins & Wheatley 1998, Blake & Ball
2001a, Ball et al. 2006, Edmunds et al. 2006). Jenkins and Wheatley (1998) documented greater variation in Heterozostera biomass among patches within a location than among locations in Port
Phillip Bay. Variation in seagrass structure among embayments has been documented, with consistently higher biomass of Z. mulleri in Port Phillip Bay than Corner Inlet or Western Port
(Ball et al. 2006).
Seagrass structure also varies through time, with significant variability among years observed in
Victoria for Heterozostera (Bulthuis & Woelkerling 1983b, Bulthuis et al. 1992, Ball et al. 2006,
Hirst et al. 2008, 2009b, a, Hirst et al. 2009c, Hirst et al. 2009d), Z. mulleri (Ball et al. 2006, Hirst et al. 2008, 2009b, a, Hirst et al. 2009c, Hirst et al. 2009d), P. australis (Roob et al. 1998, Ball et al. 2006) and A. antarctica (Edmunds et al. 2006). Seasonal variations in above-ground biomass and leaf height generally follow a unimodal pattern, with maxima in summer and minima in winter for Heterozostera (Bulthuis & Woelkerling 1983b, Ball et al. 2006, Edmunds et al. 2006), Z. mulleri (Ball et al. 2006) and A. antarctica (Edmunds et al. 2006).
Considerable spatial, temporal and interspecies variation in seagrass structure has been observed in
Victoria at a variety of scales. Variability reflects the scales investigated, methods employed, variables measured and the inherent variability in seagrass structure. The influence of variations in structure on the vulnerability, resilience, and functioning of Victorian seagrasses is unclear.
2.3
Intrinsic biological determinants of seagrass populations
2.3.1
Growth and reproduction
Seagrasses are aquatic, perennial angiosperms capable of both clonal and sexual reproduction
(Kendrick et al. 2005, Ackerman 2006). Clonal reproduction occurs via the duplication of ramets which consist of a leaf bearing shoot and a section of rhizome with roots (Kendrick et al. 2005).
Submerged flowers, filamentous pollen and submarine pollination facilitate sexual reproduction
(Ackerman 2006). Seagrasses can be dioecious or monoecious (Vermaat 2009). Seagrass leaf blades are the site of photosynthesis and can absorb nutrients, gases and water from the water column. Oxygen is transported from the leaves to roots. Roots absorb nutrients and water
(Vermaat 2009). Both acropetal and basipetal translocation of nutrients occurs. Rhizomes store carbohydrates, primarily in the form of starch and sucrose (Vermaat 2009).
Growth of Heterozostera in Port Phillip Bay was quantified by Edmunds et al. (2006) in 2004 -
2006. Aerial leaf production and stem production ranged from c.a. 150 -1000 mg dwt m -2 d -1 and
15 – 250 mg dwt m -2 d -1 , respectively. Rates of rhizome elongation and shoot production have been quantified for P. australis in New South Whales (see Meehan & West 2004).
Spadices and seeds of Heterozostera and Z. mulleri have patchy distributions in Victoria (Parry et al. 2005, Ball et al. 2006). Spadices of Heterozostera and Z. mulleri have been recorded at maximum densities of 18000 m -2 and 1300 m -2 , respectively, in Port Phillip Bay (Ball et al. 2006).
Densities of seeds were higher in the sediments of subtidal Heterozostera beds than intertidal Z. mulleri beds (Ball et al. 2006). Based on densities of spadices and seeds on plants and in sediments, the flowering season is likely to be September to January for Heterozostera, and
November to March for Z. mulleri (Parry et al. 2005). The high rates of seed production recorded for these species suggest seeds have the potential to play an important role in the maintenance and
6 Arthur Rylah Institute for Environmental Research
Review of Victorian Seagrass Research growth of seagrass beds (Parry et al. 2005). Growth and flowering intensity have not been quantified for other species in Victoria. Shoot production and flowering intensity have been quantified for some temperate seagrasses in Western Australia, including H. tasmanica and A. antarctica (see Marba & Walker 1999).
Growth rates and reproductive effort and mechanisms are largely species specific and exhibit considerable spatial and temporal variation (Marba & Walker 1999, Kendrick et al. 2005). The
‘cost’ of construction of seagrass modules increases with plant size and there is a general trend of increased rhizome elongation rates, shoot production and branching frequencies in smaller species
(Walker et al. 1999, Kendrick et al. 2005). Reproductive strategies and growth rates will influence the recovery potential of particular seagrass species following disturbance events. The role of sexual reproduction versus clonal growth in sustaining Victorian seagrass systems is presently unclear.
2.3.2
Recruitment and recovery from disturbance
The initial formation of new seagrass patches is dependant on the successful establishment of one or more propagules from seeds or rafting ramets (Kendrick et al. 2005). Subsequent clonal branching is required for the development (extent and density) of the patch. The pattern and rate of seagrass patch formation is affected by rhizome elongation and branching and shoot proliferation (Kendrick et al. 2005, Sintes et al. 2005).
Studies in Western Australia indicate comparatively rapid rhizome growth and shoot proliferation for Heterozostera ( H. tasmanica ), which was the only seagrass among those studied with the capacity to act as a pioneer species (Marba & Walker 1999). Modelling of Heterozostera patch recolonisation rates in Port Phillip Bay, based on architectural growth, indicated that a single rhizome or seedling could create a patch of c.a. 2 m 2 in 12 months (Edmunds et al. 2006).
Modelling of A. antarctica patches indicated recolonisation may take decades (Edmunds et al.
2006).
Once patches attain a critical size, seagrasses are able to alter the local environment through baffling hydrodynamic energy (see section 2.4.1) and sediment stabilisation (see section 2.4.2), leading to improved conditions for seagrass colonisation and growth. Consequently, the growth of seagrass patches may accelerate as patches increase in size (aerial extent and density, Kendrick et al. 2005). Critical patch sizes and levels of structural complexity for environmental modification by seagrasses have not been quantified in Victoria. Understanding mechanisms and rates of recruitment and patch growth is crucial to the assessment seagrass recovery following disturbance events.
2.3.3
Genetic information
Seagrass genetic variability provides information on mechanisms of recruitment, patch growth, dispersal pathways and population connectivity (Procaccini et al. 2007). Within-patch genetic diversity has been documented in Northern Hemisphere Z. marina patches, with genetic structures ranging from every ramet sampled being identical to completely unique (Olsen et al. 2004).
Genetic variation in particular seagrasses among locations has been assessed in south-western
Australia. Low genetic variation within A. antarctica among locations suggests high degrees of clonal reproduction and/or low degrees of out-breeding (Waycott et al. 1996). Moderate levels of genetic diversity have been observed for P. australis among locations spread throughout its
Australian distribution, including Corner Inlet (Waycott et al. 1997), but diversity varied
Arthur Rylah Institute for Environmental Research 7
Review of Victorian Seagrass Research depending on the analyses employed (Waycott 1998). Investigations of genetic diversity in
Victorian seagrass species at scales of 10s – 100s of kilometres have not been done.
Northern Hemisphere studies have indicated higher genotypic diversity improves resistance and resilience in P. oceania (Procaccini & Piazzi 2001) and Z. marina (Hughes & Stachowicz 2009).
Similar studies have not yet been done in the Southern Hemisphere and knowledge of the evolutionary implications of genetic diversity in seagrasses is preliminary (Procaccini et al. 2007).
Genetic monitoring may facilitate spatial and temporal tracking of populations (Procaccini et al.
2007). New genetic markers may enable understanding of stress responses of seagrasses, at the molecular level, for a range of stressors or perturbations. This may improve understanding of the selective role of stressors in adaptation (Procaccini et al. 2007).
2.4
Environmental determinants of seagrass populations
2.4.1
Hydrodynamics
Seagrass species distributions often reflect hydrodynamic regimes (Fonseca et al. 2002, Kendrick et al. 2008) with particular species, e.g. A. antarctica, occupying areas of higher flow (this is the case for Port Phillip Bay, see section 2.1). Exposure to hydrodynamic energy is widely considered an important environmental factor influencing seagrass species distributions (Kendrick et al.
2008), however, its influence on broad-scale variability compared to other mechanisms has not been empirically tested in Victoria. Hydrodynamic processes also influence the dispersal of seagrass seeds and vegetative fragments, as well as eggs and larvae of organisms that comprise seagrass communities (e.g. fish, Jenkins et al. 2000).
Seagrasses baffle unidirectional (tidal, Fonseca et al. 2002, Fonseca & Koehl 2006) and oscillatory
(wave driven, Fonseca & Cahalan 1992) currents. Plant morphology and structure affect the capacity of seagrasses to influence water flow (Verduin & Backhaus 2000, Backhaus & Verduin
2008). The capacity of seagrasses to baffle water flow is linked to the accretion of sediments (see section 2.4.1). The capacity of seagrasses to baffle currents generally increases with increasing patch structure and size (Fonseca & Koehl 2006, Backhaus & Verduin 2008). This, in turn, improves conditions for seagrass growth and recruitment, accelerating patch development (density and extent, see section 2.3.2, Kendrick et al. 2005).
The ability of seagrasses to baffle hydrodynamic currents is considered a general phenomenon.
Empirical studies of temperate seagrass responses to hydrodynamics, however, have been limited to Posidonia spp. and Amphibolis spp. in Australia (Walker et al. 1999, Verduin & Backhaus
2000).
Tidal height and range influence variability in intertidal seagrass populations, e.g. those of Z. mulleri in Victoria. Low water levels (tidal heights), barometric conditions and high temperatures can prompt prolonged atmospheric exposure and desiccation for intertidal species which may result in die-back. Atmospheric exposure and desiccation is a possible mechanism for the largescale die-back of seagrass in Western Port in the 1970s. Empirical studies on the response of seagrasses to atmospheric exposure are limited.
2.4.2
Sediments
The distributions of seagrasses in Victoria tends to reflect sediment grain size and type, with some species occupying areas typified by coarse sandy sediments (e.g. A. antarctica ) and others occupying areas of finer silts and muds (e.g. H. australis , see section 2.1). The distribution of sediments is inherently linked to hydrodynamic energy. Sediment movement prompting burial
8 Arthur Rylah Institute for Environmental Research
Review of Victorian Seagrass Research and erosion can also affect the health and distribution of seagrass species (Cabaco et al. 2008).
The resilience of seagrass species to burial and erosion is strongly size-dependent, with larger species more resilient (Cabaco et al. 2008). The relative influence of sediment characteristics and movement on broad-scale variability in seagrass distributions has not been empirically studied in
Victoria.
Seagrasses are considered to promote sediment deposition (Fonseca & Fisher 1986, Orth et al.
2006). Plant morphology and structure affects the nature of and capacity for deposition of suspended particles (including eggs, larvae and organic material) (Fonseca & Cahalan 1992). In systems dominated by tidal currents, seagrasses act as sediment sinks and contribute to the stabilisation of sediments (Walker et al. 1999) and sediment accretion can result in changes in bathymetry or ‘mounding’ in and around seagrass beds (F. Warry pers. obs.). Seagrasses are less effective sediment stabilisers in wave dominated environments (Walker et al. 1999). The capacity of seagrasses to stabilise sediments is generally thought to increase with patch structure and size
(Fonseca & Koehl 2006, Backhaus & Verduin 2008). This, in turn, improves conditions for seagrass growth and recruitment, accelerating patch development (density and extent, see section
2.3.2, Kendrick et al. 2005).
Notions of seagrasses as sediment stabilisers often arise from observations of increased turbidity following seagrass decline, as was observed in Western Port during the 1980s (Shepherd et al.
2009). Anecdotal or correlative approaches do not control for other environmental changes or relationships of cause and effect. Empirical studies in tropical Australia have shown no difference in the sediment structure and nutrient status of vegetated and unvegetated intertidal sediments (see
Mellors et al. 2002), highlighting the potential for site and species specificity in the role of seagrasses as sediment stabilisers. Comparisons of sediment stabilising attributes of seagrasses, versus other benthic habitats, in Victoria are limited, but see Bulthuis et al. (1984). Concentrations of suspended solids were higher in water ebbing from unvegetated mudflats than from
Heterozostera beds in Western Port (Bulthuis et al. 1984).
2.4.3
Light
Light influences the distribution of seagrasses. Light attenuation with depth influences the vertical extent of seagrasses, e.g. seagrasses are typically confined to depths < 20 m in Port Phillip Bay
(see section 2.1). Turbidity varies spatially and increases light attenuation, reducing the vertical extent of seagrasses, e.g. seagrasses are not found within the highly turbid waters at the Yarra
River Mouth (see section 2.1). The minimum light requirements of seagrasses typically range 2 -
37 % of surface irradiance, which is higher than those of macroalgae and phytoplankton (Lee et al.
2007 and references therin). Plant morphology influences minimum light requirements.
Halophila spp. generally have the greatest depth limits and lowest light requirements among seagrasses (this is true of the distribution of seagrasses in Port Phillip Bay; see section 2.1), which is probably a product of their oval shaped leaves and low root: shoot ratios (Lee et al. 2007 and references therein).
Light influences the condition of seagrasses. High concentrations of suspended solids and phytoplankton have been correlated with low leaf density and above ground biomass of
Heterozostera in Western Port (Campbell & Miller 2002). In situ light reduction experiments have indicated Heterozostera in Western Port requires c.a. 5 % of surface irradiance for survival, and light reduction results in decreased leaf density and death (Bulthuis 1983a). Reductions in stored carbohydrates (starches) in the rhizomes of Heterozostera in the vicinity of Port Phillip
Heads were observed during a dredging experiment in the South Channel in 2005 (Edmunds et al.
2006). This indicates photosynthesis alone may have been unable to support growth of
Arthur Rylah Institute for Environmental Research 9
Review of Victorian Seagrass Research
Heterozostera under the experimentally reduced light conditions (Edmunds et al. 2006).
Mobilisation of stored carbohydrates was not observed in A. antarctica (Edmunds et al. 2006).
Evidence exists that seagrasses can acclimate to changes in light conditions (Lee et al. 2007 and references therein). Bulthuis (1983a) demonstrated that leaf length of Heterozostera in Western
Port increased with decreasing irradiance, while growth rates remained constant possibly as a result of reduced self-shading and increased leaf surface area. Reductions in the maximum electron transport rate and light saturation point were observed for Heterozostera and A. antarctica at some sites during dredging experiments in Port Phillip Bay, indicating an adaptation to decreased irradiance possibly caused by dredge plumes (Edmunds et al. 2006). In situ light manipulation experiments have not been conducted for H. australis, P. australis or L. marina in
Victoria.
Seasonal variations in the impact of light reductions on seagrasses have been detected by Bulthuis
(1983a, Bulthuis 1983b), with more rapid declines in density in summer than winter. This may be a result of increased respiration rates during summer (see section 2.4.5, Bulthuis 1983a). The accumulation of epiphytes on seagrass leaves can vary seasonally and shade seagrasses. In
Heterozostera patches in Port Phillip Bay and Western Port, light attenuation by epiphytes showed a positive linear relationship with epiphyte biomass and significantly reduced periods of net photosynthesis (see section 4.2, Bulthuis & Woelkerling 1983a).
2.4.4
Nutrients
Nutrient regimes influence the distribution of seagrass species by affecting growth and physiology during (and after) periods of nutrient pulses, leading to reduced growth or areal decline. Adverse nutrient regimes may inhibit recruitment of seagrasses. Australian seagrasses have evolved in waters characterised by low ambient nutrient loadings. The development of seagrass patches under low-nutrient conditions is not well understood as most seagrass-nutrient research has been done in locations with high ambient nutrients (Walker et al. 1999).
Nutrient addition experiments suggest that growth of Heterozostera in Port Phillip Bay is limited by nitrogen but not phosphorous. Nitrogen enrichment of interstitial pore water resulted in significant increases in dry weight of leaves, leaf density, leaf height and concentrations of nitrogen in roots, rhizomes and leaves (Bulthuis et al. 1992). Phosphorous enrichment increased concentrations of phosphorus in Heterozostera leaves but did not alter structural parameters
(Bulthuis et al. 1992). Enrichment of interstitial pore water with ammonium and phosphorous in northern Western Port indicated that growth rates of Heterozostera are limited by nitrogen but not phosphorous, and neither ammonium nor phosphorus influences above ground biomass (Bulthuis
& Woelkerling 1981). Short term nutrient enrichment via the water column increased leaf biomass of Z. mulleri at selected sites in Western Port (Morris et al. 2008). Diel comparisons of nutrient concentrations in water ebbing from seagrass beds in Western Port indicate light dependant uptake of nitrogen by seagrasses (Bulthuis et al. 1984). Investigation of nutrient limitation and uptake has not been done for other Victorian seagrass species.
Nutrient enrichment also affects seagrass through reductions in light attenuation due to increased turbidity and shading effects from excessive algal and phytoplankton growth (see section 4.2,
Leoni et al. 2008). The source of nutrient enrichment will also influence seagrass responses. For example, nutrient enrichment via the water column results in die-off of Zostera marina , while enrichment via the sediments prompts growth (Touchette & Burkholder 2000).
Nutrient regimes can affect physiological as well as morphological characteristics of seagrasses.
Often physiological responses to nutrient enrichment occur before morphological responses (Leoni
10 Arthur Rylah Institute for Environmental Research
Review of Victorian Seagrass Research et al. 2008). Physiological responses of seagrass to nutrient enrichment include: increased chlorophyll content of leaves and simultaneous decreases in chlorophyll a/b ratios (Leoni et al.
2008); increased nitrogen and phosphorous content of leaves (Touchette 2007); increased amino acid content (Leoni et al. 2008); and, decreased carbon content in some tissues and decreased carbohydrate reserves (Leoni et al. 2008).
Predictable physiological responses to nutrient enrichment have potential as ‘nutrient pollution indicators’ (Burkholder et al. 2007) to detect stress and perturbation prior to seagrass decline.
Seagrasses have demonstrated interspecies and spatial variability in responses to nutrient enrichment (Touchette & Burkholder 2000), thus inferences from thoroughly studied species and locations should not be generalised without validation (Touchette & Burkholder 2000).
2.4.5
Water quality
Physico-chemical water properties affect seagrass physiology and growth thereby influencing seagrass species distributions. Temperature influences seasonal growth patterns in seagrasses but is difficult to separate from irradiance in situ due to longer photoperiods in summer (Lee et al.
2007). Bulthuis (1987) considered temperature the principle factor affecting seasonal growth of
Heterozostera in Victoria. Temperate seagrass species typically grow in temperatures of 11.5 – 26
ºC (Lee et al. 2007). Exposure to high temperatures generally promotes respiration relative to photosynthesis (Bulthuis 1987, Lee et al. 2007). Optimal water temperatures for photosynthesis have been found to be 30ºC for Heterozostera in Victoria (Bulthuis 1987) and 23ºC for A. antarctica in Western Australia (Masini & Manning 1997). Walker & McComb (1990) found the optimal temperature for growth of A. antarctica in Western Australia is 26ºC. The effects of temperature on growth and physiology of other Victorian seagrass species have not been explicitly investigated.
Salinity influences growth and physiology in numerous ways. For example, hypo- and hypersaline conditions can inhibit photosynthesis and nutrient uptake. Decreased soluble sugar contents of seagrass plants under high salinities may indicate conversion of carbohydrates to other organic compounds for assistance in osmotic adjustment (Touchette 2007). Studies in Western Australia have indicated that leaf production and productivity of A. antarctica are optimal in salinities of 42
(Walker 1985, Walker & McComb 1990). The effects of salinity on the growth and physiology of
Victorian seagrasses have not been explicitly investigated.
Studies on the influence of pH on seagrasses are limited. Experiments on Halophila johnsonii have demonstrated increased photosynthetic rates with decreased pH (Torquemada et al. 2005).
Similar work has not been done in Victoria.
Seagrasses have the potential to release oxygen from their roots into the surrounding sediments.
The capacity for oxygen leakage into the sediments is highly species specific (Walker et al. 1999).
Rates of oxygen release from seagrass roots have not been investigated in Victoria.
2.5
Interactions between biological and environmental factors and their influence on seagrass structure and distribution
Temporal and spatial variation in seagrass structure and distribution are likely to be the result of interactions between intrinsic biological characteristics and a suite of environmental parameters
(Figure 1). For example, interactions between mechanisms of reproduction and growth and environmental factors affecting dispersal pathways (e.g. hydrodynamics) will influence potential for recovery following disturbance.
Arthur Rylah Institute for Environmental Research 11
Review of Victorian Seagrass Research
Interactions among environmental parameters will also compound or attenuate environmental pressures on seagrass condition and distribution. For example, pulsed increases in freshwater flows (e.g. following a flood event) may locally increase nutrient concentrations and light attenuation and decrease salinity. Knowledge of how changes in these environmental parameters interact to affect seagrass condition, and ultimately distribution, is lacking.
Figure 1: Conceptual model depicting size, depth and hydrodynamic energy relationships among
Victorian seagrass species. Symbols courtesy of the Integration and Application Network
(ian.umces.edu/symbols/), University of Maryland Center for Environmental Science.
12 Arthur Rylah Institute for Environmental Research
Review of Victorian Seagrass Research
3 Seagrass communities
Seagrass beds are highly productive and support diverse communities. Organisms associated with seagrass habitats cover a wide spectrum of taxonomic groups possessing diverse life-history traits.
This is reflected in the spatial and temporal variability of algal, invertebrate, fish and bird/mammal assemblages associated with seagrass habitats.
3.1
Algae
In Victorian seagrass habitats, macroalgae can co-occur with seagrasses in mixed seagrassmacroalgae communities. Algae make an important contribution to the primary productivity of seagrass habitats and can provide habitat and nutrition (see section 4.3) for invertebrates and fish.
In Port Phillip Bay, Caulerpa remotifolia, Caulerpa brownii (Blake & Ball 2001a) and
Dictypoteric mulleri (Edmunds et al. 2006) are common microalgal species co-occurring with seagrasses, particularly Heterozostera. Seagrass patches can also accumulate drift algae, which may consist of fragments of macroalgal plants or entire plants attached to small rocks. The blades of seagrass plants provide surfaces for the colonisation of epiphytic micro- and macroalgae.
The biomass of seagrass epiphytic algae in Heterozostera habitats is typically highest in summer and lowest in winter (Bulthuis & Woelkerling 1983a). However, many species of epiphytic algae are ephemeral with appearance and biomass showing no clear seasonal pattern (Edmunds et al.
2006). This may reflect nutrient pulses (see section 4.2).
3.2
Invertebrates
Invertebrate assemblages of Victorian seagrass habitats include sessile and mobile meio-, meso- and macroinvertebrates. Meio- and mesoinvertebrate assemblages are typically dominated by small crustaceans, including amphipods, isopods, ostracods, and copepods. Macroinvertebrate assemblages include gastropods, Polychaetes, bivalves and decapods. Invasive species (e.g.
Asterias amurensis and Electroma georgiana ) occur within Victorian seagrass habitats, particularly in Port Phillip Bay.
Greater diversity and abundance of macro- (Edgar et al. 1994) and meioinvertebrates (Murphy
2007) have been recorded in seagrass ( Heterozostera ) habitats than adjacent unvegetated sediments, in Victoria. However, similar invertebrate assemblage compositions have also been reported for seagrass, Pyura and unvegetated habitats in Port Phillip Bay (Edmunds et al. 2006).
Invertebrates have been shown to respond to differences in habitat complexity beyond the presence or absence of seagrass structure (Jenkins & Sutherland 1997). Positive effects of shoot density on the abundance of harpacticoid copepods and amphipods have been recorded in Port Phillip Bay
(Jenkins & Sutherland 1997, Jenkins et al. 2002).
Invertebrate assemblages associated with Victorian seagrass habitats vary substantially in time and space. The abundance of meioinvertebrates varies within seagrass patches (Murphy 2007, Warry et al. 2009), and these patterns do not merely reflect within-patch variation in seagrass structure
(see section 4.5, Warry et al. 2009). The location of seagrass habitats within an estuary or nearshore system, and consequent differences in environmental parameters, may prompt variation in invertebrate assemblages. Bird and Jenkins (1999) attributed differences in the diversity and composition between invertebrate assemblages of seagrass habitats within Swan Bay and Port
Phillip Bay Proper to differing exposure to hydrodynamic energy.
Arthur Rylah Institute for Environmental Research 13
Review of Victorian Seagrass Research
3.3
Fish
Fish associate with seagrasses from settlement to adult life-stages (i.e. seagrass residents), and include species from the families Syngnathidae (pipefishes and seahorses), Monocanthidae
(leatherjackets), Odacidae (rock and weed whitings) and Gobiidae (Gobies) (Jenkins et al. 1997a,
Jenkins & Wheatley 1998, Hindell et al. 2000, 2001, Jenkins & Smith 2008). Syngnathids have significant conservation value, and are protected under the Environmental Protection and
Biodiversity Conservation Act, 1999. Haletta semifasciata (blue rock whiting) and meuschenia freycineti (sixspine leatherjacket) are seagrass residents of commercial value (Connolly et al.
1999).
Fish associated with seagrass for part of their lifecycle include species from the families
Sillaginidae (whitings), Platycephalidae (flatheads), Sparidae (seabreams) and Hemiramphidae
(garfishes)(Jenkins et al. 1997a, Jenkins et al. 1997b, Hindell et al. 2000, Jenkins & Hamer 2001,
Hindell 2006, Hindell et al. 2008). Several of these fish are of commercial and recreational value, including Sillaginodes punctata (king george whiting), Platycephalus laevigatus (rock flathead),
Acanthopagrus butcheri (black bream) and Hyporhamphus melanochir (southern garfish)
(Connolly et al. 1999).
Schooling fish, including species from the families Clupeoidae (sprats, herrings, sardines),
Atherinidae (hardy heads and silversides) and Arripidae (Australian salmons), often occur in the water column above seagrasses (Jenkins & Wheatley 1998, Hindell et al. 2001, Edmunds et al.
2006). These species are thought to utilise seagrass habitats for foraging (Hindell et al. 2001,
2002).
Investigations of seagrass associated fish assemblages in Victoria have historically focused on largely monospecific stands of Heterozostera and Z. mulleri in Port Phillip Bay (e.g. Jenkins et al.
1996, Jenkins et al. 1998, Jenkins & Wheatley 1998, Hindell et al. 2000, Smith et al. 2008) and to a lesser extent; Western Port (e.g. Edgar & Shaw 1995a, b, Jenkins et al. 1997b). More recently, fish assemblages have been monitored in A. antarctica patches in Port Phillip Bay (Edmunds et al.
2006, Jenkins & Smith 2008) and Heterozostera habitats in the Gippsland Lakes (Hindell & Warry
2009). Studies of fish associations with H. australis, P.australis and L. marina are limited in
Victoria.
There is generally higher fish diversity in seagrass than unvegetated habitats in Victoria (Edgar &
Shaw 1995a, Jenkins et al. 1997b). There is less consistency in trends in abundance and biomass
(Jenkins et al. 1996, Connolly et al. 1999), with similar values for seagrass and unvegetated habitats reported (Edgar & Shaw 1995a, Jenkins et al. 1997b). Higher fish diversity in seagrass than reef-algal but similar abundances in the two habitats have been recorded in Port Phillip Bay
(Jenkins & Wheatley 1998).
Spatial and temporal variation in the diversity, assemblage structure and abundance of fishes in
Victorian seagrass beds exists at a variety of scales. This may reflect seagrass structural attributes
(Jenkins & Sutherland 1997, Jenkins & Wheatley 1998) landscape configurations (Smith et al.
2008, Macreadie et al. 2009, Macreadie et al. in press ), the position of seagrass habitats within the estuary or nearshore system (Jenkins et al. 1997a, Jenkins et al. 1997b, Jenkins et al. 2000), the interaction of patch position with primary (Jenkins et al. 2000) and secondary (Hindell et al. 2003,
Moran et al. 2003, 2004b) dispersal pathways/mechanisms and temporal variability in recruitment success (Jenkins et al. 1997a).
14 Arthur Rylah Institute for Environmental Research
Review of Victorian Seagrass Research
3.4
Birds and mammals
Birds and mammals are potentially transient members of seagrass communities. The associations between birds/mammals and seagrasses (including episodic seagrass wrack) are unclear in
Victoria.
Seagrasses are known to be directly consumed by swans. Associations between seagrasses and shorebirds (Finn et al. 2007, Spruzen et al. 2008) and cormorants (Heithaus 2005) have been documented elsewhere and are thought to reflect feeding opportunities (Heithaus 2005, Finn et al.
2007).
Arthur Rylah Institute for Environmental Research 15
Review of Victorian Seagrass Research
4 Seagrass ecosystem function
4.1
Nursery paradigm
Seagrasses are widely considered a nursery habitat for fish and macroinvertebrates (Beck et al.
2001, Heck et al. 2003). The paradigm is largely based on the high diversity and productivity observed in seagrass habitats (Beck et al. 2001). Growth, survival and recruitment to adult populations have been the focus of few studies on juvenile macrofauna in seagrass habitats, despite the importance of such work being highlighted by Beck et al. (2001).
The primary value of seagrass habitats to juvenile macrofauna was thought to be due to the physical structure of seagrass affording a refuge from predation (Heck & Orth 1983). Predator exclusion experiments in Port Phillip Bay have suggested Heterozostera provides juvenile
Sillaginodes punctata with a refuge from predation (Hindell et al. 2002). However, the lack of S. punctata in the guts of the dominant piscivore in the system, Arripis truttacea , suggests that the distribution of S. punctata juveniles in seagrass-sand mosaics may be generated by processes other than direct predation (Hindell et al. 2002).
The high secondary production of seagrass habitats is considered important to juvenile macrofauna
(Beck et al. 2001). Prey distribution has been shown to influence broad-scale variability in postsettlement S. punctata among seagrass habitats in Port Phillip Bay (Jenkins & Hamer 2001). The distribution of small Syngnathid fishes within seagrass habitats in Port Phillip Bay has also been shown to be related to food availability (Macreadie et al. in press ). There is a growing body of evidence to suggest that the value of seagrass habitats, including those in Port Phillip Bay, to juvenile macrofauna relates to the position of seagrass beds within an estuary, and thus proximity to larval influxes (Bell et al. 1988, Jenkins et al. 1996, Jenkins et al. 1998).
The movement of juveniles from seagrass to adult habitats has not been quantified in Victoria, relative to other juvenile habitats. New tagging techniques (e.g. calcein, Smith et al. in press-a ) and otolith microchemistry (Gillanders 2002, Gillanders & Kingsford 2003) may be useful in investigating the contribution of juveniles from seagrass habitats to adult populations.
4.2
Catchment-seagrass links
Seagrasses are impacted by catchment land use and hydrology. Nutrients and sediments are prominent components of catchment-derived inputs to estuarine and nearshore environments and can alter seagrass ecosystem structure and function.
Assimilation of terrestrial nitrogen into seagrass plants and subsequent incorporation into nearshore food webs has been demonstrated using stable isotopes in Anderson Inlet (for
Heterozostera , Hindell 2008) and Corner Inlet (for H. australis, P. australis and Heterozostera ,
Hindell et al. 2009). In Corner Inlet, nitrogen isotope signatures implicated sewage effluent as a potential source of nitrogen, and ‘contamination’ of seagrass and fish was system-wide (Hindell et al. 2009).
Species interactions within seagrass communities may change with altered nutrient regimes
(Burkholder et al. 2007). The nature of species interactions will depend on the source of nutrient enrichment e.g. via the water column versus the sediments (Burkholder et al. 2007, Touchette
2007). Nutrient enrichment via the water column is considered most detrimental to seagrass health and the most likely mechanism of nutrient enrichment in Victorian systems (Morris et al. 2007).
Microalgae have higher rates of nutrient absorption than macroalgae, which in turn have higher rates than seagrasses. In addition, algae typically have lower light requirements than seagrasses
(Burkholder et al. 2007). The role of rapid and excessive growth of epiphytes, macroalgae and
16 Arthur Rylah Institute for Environmental Research
Review of Victorian Seagrass Research phytoplankton, leading to increased shading of seagrasses and subsequent reduced photosynthetic capacities, is considered a prominent mechanism of seagrass decline (Burkholder et al. 2007,
Morris et al. 2007).
Short-term experimental nutrient enrichment of the water column above Z. mulleri plants in
Western Port, increased leaf and loose algal biomass at some sites (Morris et al. 2007). Increased densities of amphipods at some sites may mitigate nutrient enrichment to some degree through grazing algae (Morris et al. 2007). The longer-term impacts of nutrient enrichment in Victoria remain uncertain, particularly the recovery potential of seagrasses that have experienced stress or decline and assessment of environmental conditions under which recovery may occur.
A suite of indirect effects of catchment-derived nutrient and sediment inputs can accelerate seagrass decline. These include: sediment re-suspension from seagrass decline and subsequent erosion and turbidity; thick macroalgae impeding hydrodynamic flushing thus preventing dilution of nutrients and sediments and delivery of oxygen; increased system respiration leading to hypoxic and anoxic conditions and; biogeochemical alterations of the sediment (Burkholder et al. 2007).
Interactions between indirect stressors imposed by nutrient enrichment (or eutrophication) and environmental conditions will determine the ultimate impacts of nutrient enrichment on seagrass health and function, but are poorly understood.
4.3
Trophic Ecology
Seagrasses are thought to be important in supporting nearshore food webs (Moran et al. 2004a,
Connolly et al. 2005b, Hindell 2006). Seagrass can provide a base for nutritional support through detrital (Connolly et al. 2005a, Melville & Connolly 2005) and direct grazing (Valentine & Duffy
2006) pathways. Trophic subsidies from seagrasses have been documented for mudflats (Connolly et al. 2005a), mangroves (Marguillier et al. 1997), coral reefs (Nagelkerken & van der Velde
2004a, b) and terrestrial systems (Heck et al. 2008 and references therein). Although there have been numerous qualitative assessments of the importance of seagrass detrital export and assimilation into food webs (see Heck et al. 2008), there have been relatively few quantitative assessments of the magnitude of detrital fluxes and only one from the Southern Hemisphere (see
Kirkman & Reid 1979 on P. australis in NSW).
Stable isotope studies have shown Heterozostera to contribute to the nutrition of early post-larval
S. punctata in Port Phillip Bay (Moran et al. 2004a). Additionally, Heterozostera was putatively the greatest contributor (> 50 %) to the nutritional base of the piscivores Platycephalus speculator and Platycephalus laevigatus , but its contribution to the nutrition of A. truttacea was unclear
(Hindell 2006). Seagrass has also been shown to be a likely base for nutritional support of two decapod species ( Biffarius arenosus and Tryyea australiensis ) in Western Port (Boon et al. 1997).
Seagrasses were putatively the dominant contributor to the nutrition of S. punctata in Corner Inlet , while seagrass epiphytes were more important for the nutrition of H. melanochir and A. fosteri
(Hindell et al. 2009). The relative contribution of P. australis, H. australis and Heterozostera to food webs in Corner Inlet could not be clearly separated using carbon and nitrogen stable isotopes
(Hindell et al. 2009). Sulphur isotopes (Connolly et al. 2004) and manipulation of isotopic signatures (Winning et al. 1999) may allow differentiation of seagrass species. Amphibolis spp. and Posidonia spp. have been shown to contribute to nearshore food webs in Western Australia
(Hyndes & Lavery 2005).
The exact nature of pathways through which seagrass is incorporated into food webs (i.e. detrital vs. trophic relay) in Victorian nearshore systems remains unclear, and trophic subsidies between seagrasses and other nearshore habitats are poorly understood. In addition, the trophic interactions
Arthur Rylah Institute for Environmental Research 17
Review of Victorian Seagrass Research between seagrasses and terrestrial systems have not been studied in Victoria. In particular, the role of seagrass wrack in coastal food webs remains unclear, despite its prevalence in Victorian nearshore systems. The status of trophic dynamics in nearshore systems (and the contribution of seagrass) under different population (e.g. seagrass ramet age structure), environmental (e.g. nutrient enrichment) and landscape (e.g. habitat fragmentation) scenarios is unclear.
4.4
Carbon cycling and sequestration
Seagrass systems have very high rates of primary production (Orth et al. 2006) and are generally considered to be net autotrophic (Mateo et al. 2006, Heck et al. 2008). Rates of primary production of seagrasses and the fate of seagrass-derived organic carbon is highly variable, and probably species specific (Mateo et al. 2006, Heck et al. 2008). Mateo et al. (2006) suggested c.a.
65 % of seagrass production is decomposed within a bed, c.a. 15 % is exported, < 10 % is grazed and c.a. 10 % accumulates in a refractory pool (i.e. carbon burial). Although carbon export and burial represent modest proportions relative to other fates of seagrass organic carbon, the extreme productivity of seagrass systems means that this actually represents a large quantity of carbon flux
(Mateo et al. 2006).
Seagrass-derived organic carbon can be exported in the form of detritus or dissolved organic material (Mateo et al. 2006, Heck et al. 2008). Seagrass detrital export is important in the global ocean carbon cycle by subsidising the high respiratory requirements of pelagic ecosystems, which tend to be net heterotrophic (Duarte et al. 2005 and references therein). Seagrasses excrete dissolved organic carbon (DOC) from living leaves, roots and rhizomes; DOC also leaches from decomposing tissues. Seagrasses are considered net sources of DOC to both the water column and sediment interstitial waters (Mateo et al. 2006 and references therein). The relative importance and fates of seagrass-derived DOC in nutrient cycling and secondary production are yet to be quantified (Mateo et al. 2006).
Refractory carbon refers to any excess component once all known fates of organic material have been accounted for (Mateo et al. 2006). Once refractory carbon is sequestered or buried it remains in its organic form; resistant to decomposition. Seagrasses can act as short term sinks with residence times typically in the order of 1 - 2 years for above-ground biomass, or 4 – 6 years for below-ground biomass. Seagrasses can also act as long term sinks (or reservoirs) where refractory carbon is buried and stored in a form whereby the biomass and nutrient content of the material remains constant over time-scales of millennia (Mateo et al. 2006). Factors influencing the burial of refractory carbon in below-ground seagrass tissues include; processes of material accumulation, chemistry of the refractory material and the redox potential and anoxia of the sediments (Mateo et al. 2006).
Worldwide there is relatively little known about the nature of carbon sequestration in seagrasses and their roles as carbon sinks. The rate and magnitude of carbon export from Victorian seagrass systems is unclear. The potential of various Victorian seagrass species to act as carbon sinks and sequester carbon dioxide is presently unknown. Due to the value of seagrasses as net autotrophic carbon sinks, the impacts of seagrass decline are likely to reach beyond immediate loss in biodiversity to compromised function of estuarine and marine ecosystems.
4.5
Landscape ecology
Seagrass landscape ecology has typically focused on seagrass-sand habitats whereby seagrass patches are configured within a matrix of unvegetated sand or vice versa (Bostrom et al. 2006,
18 Arthur Rylah Institute for Environmental Research
Review of Victorian Seagrass Research
Connolly & Hindell 2006). Fish and meioinvertebrates have demonstrated positive (Smith et al.
2008, Macreadie et al. 2009, Warry et al. 2009), negative (Smith et al. 2008, in press-b ) and no responses (Warry et al. 2009) to Heterozostera patch edges (or artificial seagrass modelled on the physical structure of Heterozostera ) in Port Phillip Bay. The magnitude of edge effects in
Syngnathid fishes and harpacticoid copepods varies with patch size (Smith et al. in press-b ) and habitat fragmentation (Warry et al. 2009), respectively. Food availability is a likely mechanism eliciting edge effects for Syngnathids (Macreadie et al. in press ). The configuration of seagrass patches can also affect species diversity, with more species of fish in continuous and historically continuous patches than patchy configurations (Macreadie et al. 2009).
There has been much less focus on more complex habitat mosaics, consisting of multiple habitat types such as seagrasses, reefs, and mangroves (see Bostrom et al. 2006, Connolly & Hindell
2006). The impacts of the spatial arrangement of seagrasses in relation to other structured habitats
(e.g. reefs) on ecological patterns and processes are unclear in Victoria. To date, seagrass landscape studies in Victoria have investigated landscape effects on patterns in abundance, density, diversity and assemblage composition and possible mechanisms generating these patterns.
Landscape effects on seagrass ecosystem function, such as trophic dynamics and nutrient cycling, have not yet been addressed.
Arthur Rylah Institute for Environmental Research 19
Review of Victorian Seagrass Research
5 Management
Research is an important component of seagrass management. Identification and understanding of threats to seagrass and monitoring and assessment of seagrass condition are crucial to the prevention and mitigation of adverse impacts of disturbance. Techniques for restoration and rehabilitation offer managers tools to address seagrass decline.
5.1
Threats to seagrass
The major threats to seagrass in Victoria (and worldwide) stem from population pressures in the coastal zone, and seagrasses are likely to be increasingly threatened under projected population growth and climate change scenarios (Orth et al. 2006, Waycott et al. 2009). Likely threats to seagrass stemming from population pressures include:
Greater sediment and nutrient loads entering nearshore systems;
Mechanical damage from commercial (e.g. dredging, commercial fishing techniques) and recreational (e.g. propeller scaring, anchor damage, trampling) activities;
Marine pest introductions;
Inputs from aquaculture operations; and,
Alterations of hydrodynamic regimes (e.g. regulation of freshwater flows, alteration of nearshore circulation by built structures and modification of bathymetry).
Interactions among these threats are complex and not fully understood. Climate change represents an additional broad-scale threat to seagrass ecosystems. Threats to seagrass ecosystems stemming from climate change are likely to be exacerbated by population growth and may include:
Higher atmospheric and water temperatures;
Sea level rise, particularly where coastal development impedes shoreline evolution;
Alteration of ocean chemistry, particularly ocean acidification;
Alterations in the frequency and severity of storm events;
Changes in circulation patterns of oceanic and nearshore waters;
Changes in inputs of flows, nutrients and sediments from catchments;
Alteration of biological interactions.
The exact nature of seagrass responses to different threats is likely to be species specific and will depend on the intrinsic biological and environmental determinants of growth for a particular species. Understanding how threats interact will improve prediction of the ultimate impacts of population pressures and climate change on seagrass condition which will lead to more effective management of seagrasses.
5.2
Assessing seagrass condition
Conventional metrics of seagrass health have focused on physical attributes of the seagrass plants.
In Victoria these have included areal extent (e.g. Blake & Ball 2001a, b, Ball et al. 2006), leaf density (e.g. Blake & Ball 2001a, b, Hirst et al. 2009d), seagrass epiphytic cover (e.g. Ball et al.
2006, Hindell & Warry 2009), seed and flower densities (Ball et al. 2006) and shoot and rootrhizome biomass (Ball et al. 2006). Measurement of these attributes is relatively affordable and efficient, particularly those attributes that can be mapped remotely e.g. areal extent. However, the value of monitoring physical attributes of seagrass is questionable given inherent variability in physical attributes and the likelihood that seagrass decline may occur before a change is noticed.
20 Arthur Rylah Institute for Environmental Research
Review of Victorian Seagrass Research
Environmental parameters known to influence seagrass growth, including light penetration/turbidity and temperature have been monitored (e.g. Ball et al. 2006), however, such approaches are only valuable if species-specific responses and critical thresholds are known.
Monitoring physiological characteristics of seagrasses has the potential to detect stress in seagrass plants before decline occurs. In Victoria, the mobilisation of stored carbon (starch) has been used as a metric for seagrass stress as a result of low light availability (see section 2.4.3, Edmunds et al.
2006). Other potentially useful physiological metrics of seagrass health that could be utilized to detect stress responses to threats associated with sedimentation and nutrient enrichment include: nitrogen and phosphorus contents of leaves; amino acid content of tissues; chlorophyll contents; and, chlorophyll a/b ratios (see section 2.4.4, Touchette 2007, Leoni et al. 2008). These emerging metrics are yet to be critically evaluated in the Victorian context.
A Victorian Index for Estuarine Condition (IEC) has been developed to facilitate a consistent state-wide assessment of estuarine condition, prioritisation of resource allocation and strategic evaluation of management interventions (Arundel et al. 2009). The index is comprised of six themes (physical form, hydrology, water quality, sediment, flora and fauna), and acknowledges seagrasses as an important component of estuarine flora (macrophytes) contributing to estuarine function. The sampling protocols and scoring description for macrophytes are still under development (Arundel et al. 2009), however, assessments will likely be based on physical attributes. The IEC will help to assess seagrass condition from an ecosystem (estuarine) based perspective.
To date, Victorian assessments of seagrass condition have focused on physical properties and physiology of the seagrass plants themselves. However, the relative influence of seagrass condition on ecological processes such as trophic dynamics, carbon and nutrient cycling remains unclear.
5.3
Options for addressing seagrass decline
Seagrass restoration and rehabilitation through seeding (Orth et al. 2006) and transplantation of seedlings (Paling et al. 2007) is an option for restoring seagrass habitat where the underlying environmental issues that first led to seagrass decline have been resolved. Maximising the success of seagrass restoration relies on reversal of the habitat degradation or cause of seagrass decline, selection of appropriate habitats based on species specific requirements, selection of appropriate donor populations (including sufficient genetic diversity) and spreading the risks of recruitment failure in space and time (van Katwijk et al. 2009). Although a growing body of work on seagrass restoration techniques exists, rates of recruitment, growth and survival of seeds and transplanted seedlings are notoriously variable (van Katwijk et al. 2009) and techniques are often expensive and labour intensive (Elliott et al. 2007, Paling et al. 2007, Orth et al. 2009, van Katwijk et al. 2009).
A review of restoration techniques by Parry (2007) identified restoration using seeds as the most cost-efficient method, but acknowledged the technique is not appropriate in all situations.
Techniques developed in the United States for Zostera marina may be valid for Z. mulleri and
Heterozostera in Victoria (Parry 2007), however, better knowledge of seagrass ecology
(particularly the ecology of seeds) is necessary to confidently assess the feasibility of Victorian restoration programs (Parry et al. 2005, Parry 2007).
A major obstacle to the re-colonisation of seagrass following decline or loss is the destabilisation of sediments (see section 2.4.2, Collings 2008, van Katwijk et al. 2009). Collings et al. (2007,
2008) have used Hessian recruitment units (Hessian bags filled with sand) to enhance recruitment of Amphibolis antarctica in South Australia. The Hessian recruitment units provide an anchoring
Arthur Rylah Institute for Environmental Research 21
Review of Victorian Seagrass Research point for the ‘grappling hook’ apparatus of A. antarctica seedlings to attach to. Although results have been variable, the technique shows promise for broad-scale rehabilitation of A. antarctica
(Collings et al. 2007, Collings 2008). Interventions that promote seagrass recruitment may provide a more cost-effective alternative to seagrass transplantation and seeding.
To date, trials of seagrass seeding, transplantation or recruitment enhancement techniques have not occurred in Victoria. Although such techniques may prove effective for restoration and rehabilitation of seagrass habitats, managing adverse environmental conditions to minimise seagrass decline while promoting seagrass resilience (the ability of seagrass systems to attain stability following disturbance) will ultimately be the most successful and cost-effective approach to seagrass management (Elliott et al. 2007, Parry 2007).
22 Arthur Rylah Institute for Environmental Research
Review of Victorian Seagrass Research
6 Summary Points
The majority of seagrass research in Victoria has focused on Heterozostera and Z. mulleri.
Recently, A. antarctica has been included in environmental effects statements and monitoring programs as part of the Channel Deepening Project in Port Phillip Bay.
Current Victorian seagrass maps are primarily based on ground truthed aerial photography.
Areal extent has been combined with metrics of condition, including physical structure, seed and flower densities and epiphytic cover, at certain sites.
Seagrass physical structure varies at multiple spatio-temporal scales and has been shown to influence the composition and distribution of fish and invertebrate assemblages.
Seeds and flowers of Heterozostera, Z. mulleri and P. australis are patchily distributed, however, high rates of seed production suggests seeds have the potential to play an important role in the maintenance and growth of Victorian seagrass beds.
Modelled recolonisation rates of Heterozostera exceed those of A. antarctica , and
Heterozostera may have the capacity to act as a pioneer seagrass species.
Little genetic information is available for Victorian seagrasses, particularly the role of genetic diversity in resistance and resilience.
Victorian seagrass species’ distributions reflect hydrodynamic regimes, sediment grain size, light attenuation and depth.
The effect of particular physico-chemical water quality parameters on the physiology of
Victorian seagrass species is unclear.
Plant morphology influences minimum light requirements. Heterozostera and A. antarctica have shown physical and physiological responses to experimental light reduction.
Nutrient manipulation experiments have demonstrated nitrogen, but not phosphorous, limits growth of Heterozostera in Port Phillip Bay and Western Port.
Seagrasses support diverse algal and faunal communities that have been surveyed relatively extensively for Heterozostera and Z. mulleri but less so for other Victorian seagrass species.
The value of Victorian seagrasses to small and juvenile fish and macroinvertebrates is thought to reflect food availability and the position of seagrass habitats within estuaries rather than seagrass acting as a refuge from predation.
Rapid micro- and macroalgal growth and subsequent shading effects under enriched nutrient regimes is considered a prominent mechanism of seagrass decline. Amphipods, macroalgae and Z. mulleri have responded positively to short term experimental nutrient enrichment via the water column in Victoria.
Assimilation of terrestrial nitrogen into seagrass plants and subsequent incorporation into nearshore food webs has been documented in Victoria.
Victorian seagrasses have been shown to provide the nutritional base for fish and decapods, however, it has been difficult to separate the contribution of individual seagrass species.
The capacity of Victorian seagrass species to act as carbon sinks and sequester carbon dioxide is unknown.
Habitat fragmentation and edge effects can influence the distribution and diversity of fish and invertebrates in Heterozostera and artificial seagrass modelled on Heterozostera .
Prominent threats to seagrass stem from population pressures in the coastal zone and climate change.
Arthur Rylah Institute for Environmental Research 23
Review of Victorian Seagrass Research
In Victoria, seagrass condition has typically been assessed by monitoring physical properties of seagrass and environmental conditions thought to affect seagrass health.
Restoration and rehabilitation through seeding, transplantation of seedlings and recruitment enhancement has not been trialled in Victoria.
Key ecological relationships in normal and modified seagrass ecosystems are summarised in figures 2 and 3 respectively.
Figure 2: Conceptual model depicting key ecological relationships in unmodified seagrass ecosystems.
Symbols courtesy of the Integration and Application Network (ian.umces.edu/symbols/), University of
Maryland Center for Environmental Science.
24 Arthur Rylah Institute for Environmental Research
Review of Victorian Seagrass Research
Figure 3: Conceptual model depicting key ecological relationships in modified seagrass ecosystems.
Symbols courtesy of the Integration and Application Network (ian.umces.edu/symbols/), University of
Maryland Center for Environmental Science.
Arthur Rylah Institute for Environmental Research 25
Review of Victorian Seagrass Research
References
Ackerman JD (2006) Sexual reproduction of seagrasses: pollination in the marine context. In:
Larkum WDA, Orth RJ, Duarte CM (eds) Seagrasses: Biology, Ecology and Conservation.
Springer, Dordrecht, The Netherlands, p 89-109
Arundel H, Pope A, Quinn G (2009) Victorian Index of Estary Condition. Recommended themes and measures. Draft Report., School of Environmental Sciences, Deakin University
Backhaus JO, Verduin JJ (2008) Simulating the interaction of seagrasses with their ambient flow.
Estuar Coast Shelf Sci 80:563-572
Ball D, Parry GD, Heislers S, Blake S, Werner G (2006) Analysis of Victorian seagrass health at a multi-regional level. Progress Report 1. Internal Report No. 83. Primary Industries
Research Victoria, Queenscliff
Beck MW, Heck KL, Able KW, Childers DL, Eggleston DB, Gillanders BM, Halpern B, Hays
CG, Hoshino K, Minello TJ, Orth RJ, Sheridan PF, Weinstein MR (2001) The identification, conservation, and management of estuarine and marine nurseries for fish and invertebrates. Bioscience 51:633-641
Bell JD, Steffe AS, Westoby M (1988) Location of Seagrass Beds in Estuaries - Effects on
Associated Fish and Decapods. J Exp Mar Biol Ecol 122:127-146
Bird FL, Jenkins G (1999) Abundance, biomass and estimated production of invertebrate fauna associated with seagrass, Heterozostera tasmanica , in Swan Bay and an adjacent area of
Port Phillip Bay, Victoria. Proceedings of the Royal Society of Victoria 111:1-13
Blake S, Ball D (2001a) Victorian Marine Habitat Database: seagrass mapping of Port Phillip Bay.
Geospatial Systems Section Marine and Freshwater Resources Institute Report No. 39,
Marine and Freshwater Resources Institute, Queenscliff
Blake S, Ball D (2001b) Victorian Marine Habitat Database: Seagrass Mapping of Western Port.
Geospatial Systems Section, Marine and Freshwater Resources Institute Report No. 29,
(Marine and Freshwater Resources Institute: Queenscliff)
Blake S, Roob R, Patterson E (2000) Seagrass Mapping of Victoria’s Minor Inlets. Marine and
Freshwater Resources Institute Report No. 28. July 2000, (Marine and Freshwater
Resources Institute: Queenscliff)
Boon PI, Bird FL, Bunn SE (1997) Diet of the intertidal callianassid shrimps Biffarius arenosus and Trypea australiensis (Decapoda : Thalassinidea) in Western Port (southern Australia), determined with multiple stable-isotope analyses. Mar Freshw Res 48:503-511
Bostrom C, Jackson EL, Simenstad CA (2006) Seagrass landscapes and their effects on associated fauna: A review. Estuar Coast Shelf Sci 68:383-403
Bulthuis DA (1983a) Effects of Insitu Light Reduction on Density and Growth of the Seagrass
Heterozostera-Tasmanica (Martens Ex Aschers) Den Hartog in Western Port, Victoria,
Australia. J Exp Mar Biol Ecol 67:91-103
Bulthuis DA (1983b) Effects of Temperature on the Photosynthesis Irradiance Curve of the
Australian Seagrass, Heterozostera-Tasmanica. Marine Biology Letters 4:47-57
Bulthuis DA (1987) Effects of Temperature on Photosynthesis and Growth of Seagrasses. Aquat
Bot 27:27-40
Bulthuis DA, Axelrad DM, Mickelson MJ (1992) Growth of the Seagrass Heterozostera-
Tasmanica Limited by Nitrogen in Port Phillip Bay, Australia. Mar Ecol-Prog Ser 89:269-
275
Bulthuis DA, Brand GW, Mobley MC (1984) Suspended Sediments and Nutrients in Water
Ebbing from Seagrass-Covered and Denuded Tidal Mudflats in a Southern-Australian
Embayment. Aquat Bot 20:257-266
Bulthuis DA, Woelkerling WJ (1981) Effects of Insitu Nitrogen and Phosphorus Enrichment of the
Sediments on the Seagrass Heterozostera-Tasmanica (Martens Ex Aschers) Denhartog in
Western Port, Victoria, Australia. J Exp Mar Biol Ecol 53:193-207
Bulthuis DA, Woelkerling WJ (1983a) Biomass Accumulation and Shading Effects of Epiphytes on Leaves of the Seagrass, Heterozostera-Tasmanica, in Victoria, Australia. Aquat Bot
16:137-148
26 Arthur Rylah Institute for Environmental Research
Review of Victorian Seagrass Research
Bulthuis DA, Woelkerling WJ (1983b) Seasonal-Variation in Standing Crop, Density and Leaf
Growth-Rate of the Seagrass, Heterozostera-Tasmanica, in Western Port and Port Phillip
Bay, Victoria, Australia. Aquat Bot 16:111-136
Burkholder JM, Tomasko DA, Touchette BW (2007) Seagrasses and eutrophication. J Exp Mar
Biol Ecol 350:46-72
Cabaco S, Santos R, Duarte CM (2008) The impact of sediment burial and erosion on seagrasses:
A review. Estuar Coast Shelf Sci 79:354-366
Campbell SJ, Miller CJ (2002) Shoot and abundance characteristics of the seagrass Heterozostera tasmanica in Westernport estuary (south-eastern Australia). Aquat Bot 73:33-46
Collings GJ (2008) Seagrass rehabilitation in metropolitan Adelaide V. Large scale recruitment trial, Prepared for the Coastal Protection Branch, Department for Environment and
Heritage. South Australian Research and Development Institute (Aquatic Sciences),
Adelaide, 32pp. SARDI Publication No. F2008/000077
Collings GJ, Venema S, Wear RJ, Tanner JE (2007) Seagrass rehabilitation in metropolitan
Adelaide IV. Geographic and interannual variability of recruitment facilitation., Prepared for the Coastal Protection Branch, Department for Environment and Heritage. SARDI
Aquatic Sciences Publication No. 2007/000268-1 SARDI Aquatic Sciences, Adelaide
Connolly R, Jenkins G, Loneragan N (1999) Seagrass dynamics and fisheries sustainability. In:
Butler A, Jernakoff P (eds) Seagrass in Australia. CSIRO Publishing, Collingwood,
Australia, p 23-64
Connolly RM, Gorman D, Guest MA (2005a) Movement of carbon among estuarine habitats and its assimilation by invertebrates. Oecologia 144:684-691
Connolly RM, Guest MA, Melville AJ, Oakes JM (2004) Sulfur stable isotopes separate producers in marine food-web analysis. Oecologia 138:161-167
Connolly RM, Hindell JS (2006) Review of nekton patterns and ecological processes in seagrass landscapes. Estuar Coast Shelf Sci 68:433-444
Connolly RM, Hindell JS, Gorman D (2005b) Seagrass and epiphytic algae support nutrition of a fisheries species, Sillago schomburgkii, in adjacent intertidal habitats. Mar Ecol-Prog Ser
286:69-79
Duarte CM, Middelburg JJ, Caraco N (2005) Major role of marine vegetation on the oceanic carbon cycle. Biogeosciences 2:1-8
Edgar GJ, Shaw C (1995a) The production and trophic ecology of shallow-water fish assemblages in southern Australia .1. Species richness, size-structure and production of fishes in
Western Port, Victoria. J Exp Mar Biol Ecol 194:53-81
Edgar GJ, Shaw C (1995b) The production and trophic ecology of shallow-water fish assemblages in southern Australia .2. Diets of fishes and trophic relationships between fishes and benthos at Western Port, Victoria. J Exp Mar Biol Ecol 194:83-106
Edgar GJ, Shaw C, Watson GF, Hammond LS (1994) Comparisons of Species Richness, Size-
Structure and Production of Benthos in Vegetated and Unvegetated Habitats in Western-
Port, Victoria. J Exp Mar Biol Ecol 176:201-226
Edmunds M, Pickett P, Stewart K (2006) Port Phillip Bay Channel Deepening Project
Supplementary Environmental Effects Statement - Marine Ecology Specialist Studies.
Volume 5: Seagrass Beds. Report to Port of Melbourne Corporation and Maunsell. Report
No. 354, Australian Marine Ecology, Melbourne
Elliott M, Burdon D, Hemingway KL, Apitz SE (2007) Estuarine, coastal and marine ecosystem restoration: Confusing management and science - A revision of concepts. Estuar Coast
Shelf Sci 74:349-366
Environment DoCa (1991) Swan Bay Marine and Wildlife Reserves. Proposed Management Plan.
Department of Conservation and Environment.
Finn PG, Catterall CP, Driscoll PV (2007) Determinants of preferred intertidal feeding habitat for
Eastern Curlew: A study at two spatial scales. Austral Ecol 32:131-144
Fonseca M, Fisher JS (1986) Comparison of canopy friction and sediment movement between four species of seagrass with reference to their ecology and restoration. Mar Ecol-Prog Ser
29:15-22
Arthur Rylah Institute for Environmental Research 27
Review of Victorian Seagrass Research
Fonseca M, Whitfield PE, Kelly NM, Bell SS (2002) Modeling seagrass landscape pattern and associated ecological attributes. Ecol Appl 12:218-237
Fonseca MS, Cahalan JA (1992) A Preliminary Evaluation of Wave Attenuation by 4 Species of
Seagrass. Estuar Coast Shelf Sci 35:565-576
Fonseca MS, Koehl MAR (2006) Flow in seagrass canopies: The influence of patch width. Estuar
Coast Shelf Sci 67:1-9
Fyfe SK (2003) Spatial and temporal variation in spectral reflectance: Are seagrass species spectrally distinct? Limnology and Oceanography 48:464-479
Gillanders BM (2002) Connectivity between juvenile and adult fish populations: do adults remain near their recruitment estuaries? Mar Ecol-Prog Ser 240:215-223
Gillanders BM, Kingsford MJ (2003) Spatial variation in elemental composition of otoliths of three species of fish (family Sparidae). Estuar Coast Shelf Sci 57:1049-1064
Heck KL, Carruthers TJB, Duarte CM, Hughes AR, Kendrick G, Orth RJ, Williams SW (2008)
Trophic Transfers from Seagrass Meadows Subsidize Diverse Marine and Terrestrial
Consumers. Ecosystems 11:1198-1210
Heck KL, Hays G, Orth RJ (2003) Critical evaluation of the nursery role hypothesis for seagrass meadows. Mar Ecol-Prog Ser 253:123-136
Heck KL, Orth RJ (1983) Predator-Prey Relationships in Seagrass Ecosystems - a Reexamination of Hypotheses. Estuaries 6:256-256
Heithaus MR (2005) Habitat use and group size of pied cormorants (Phalacrocorax varius) in a seagrass ecosystem: possible effects of food abundance and predation risk. Mar Biol
147:27-35
Hindell JS (2006) Assessing the trophic link between seagrass habitats and piscivorous fishes. Mar
Freshw Res 57:121-131
Hindell JS (2008) Base for nutritional support of estuary perch in Anderson Inlet: evaluating the relative importance of Spartina. Arthur Rylah Institute for Environmental Research
Technical Report Series No. 2008/60, Department of Sustainability and Environment:
Heidelberg
Hindell JS, Ball D, Brady B, Hatton D (2009) Assesment of the estuarine water quality and its effects on seagrass health in Corner Inlet. Fisheries Victoria Technical Report No. 46, 42 pages, Department of Primary Industries, Queenscliff, Victoria, Australia
Hindell JS, Jenkins GP, Keough MJ (2000) Variability in abundances of fishes associated with seagrass habitats in relation to diets of predatory fishes. Mar Biol 136:725-737
Hindell JS, Jenkins GP, Keough MJ (2001) Spatial and temporal variability in the effects of fish predation on macrofauna in relation to habitat complexity and cage effects. Mar Ecol-Prog
Ser 224:231-250
Hindell JS, Jenkins GP, Keough MJ (2002) Variability in the numbers of post-settlement King
George whiting (Sillaginidae : Sillaginodes punctata, Cuvier) in relation to predation, habitat complexity and artificial cage structure. J Exp Mar Biol Ecol 268:13-31
Hindell JS, Jenkins GP, Moran SM, Keough MJ (2003) Swimming ability and behaviour of postlarvae of a temperate marine fish re-entrained in the pelagic environment. Oecologia
135:158-166
Hindell JS, Jenkins GP, Womersley B (2008) Habitat utilisation and movement of black bream
Acanthopagrus butcheri (Sparidae) in an Australian estuary. Mar Ecol-Prog Ser 366:219-
229
Hindell JS, Warry FY (2009) Fish assemblages and seagrass condition of the Gippsland Lakes,
Arthur Rylah Institute for Environmental Research. Department of Sustainability and
Environment, Heidelberg, Victoria
Hirst A, Ball D, Heislers S, Blake S, Coots A (2008) Baywide Seagrass Monitoring Program,
Milestone Report No. 1 (2008) - Ed. 2. Fisheries Victoria Technical Report No. 25,
January 2009, Department of Primary Industries, Queenscliff, Victoria, Australia. 30pp.
Hirst A, Ball D, Heislers S, Blake S, Coots A (2009a) Baywide Seagrass Monitoring Program,
Milestone Report No. 3 (2008). Fisheries Victoria Technical Report Series No. 39, March
2009, Department of Primary Industries, Queenscliff, Victoria, Australia. 38pp.
28 Arthur Rylah Institute for Environmental Research
Review of Victorian Seagrass Research
Hirst A, Ball D, Heislers S, Blake S, Coots A (2009b) Baywide Seagrass Monitoring Program,
Milestone Report No. 4 (Jan-Feb 2009). Fisheries Victoria Technical Report Series No.
43, April 2009, Department of Primary Industries, Queenscliff, Victoria, Australia. 44pp.
Hirst A, Ball D, Heislers S, Young P, Blake S, Coots A (2009c) Baywide Seagrass Monitoring
Program, Milestone Report No. 2 (2008). Fisheries Victoria Technical Report No. 29,
January 2009., Department of Primary Industries, Queenscliff, Victoria, Australia. 48pp.
Hirst A, Heislers S, Ball D, Blake S, Coots A (2009d) Baywide Seagrass Monitoring Program,
Milestone Report No. 5 (April-May 2009). Fisheries Victoria Technical Report Series No.
63, August 2009, Department of Primary Industries, Queenscliff, Victoria, Australia.
46pp.
Hughes AR, Stachowicz JJ (2009) Ecological impacts of genotypic diversity in the clonal seagrass
Zostera marina. Ecology 90:1412-1419
Hyndes GA, Lavery PS (2005) Does transported seagrass provide an important trophic link in unvegetated, nearshore areas? Estuar Coast Shelf Sci 63:633-643
Ierodiaconou D, Burq S, Reston M, Laurenson L (2007) Marine benthic habitat mapping using multibeam data, georeferenced video and image classification techniques in Victoria,
Australia. Spatial Sciences Inst, p 93-104
Ierodiaconou DA, Laurenson LJB (2002) Estimates of Heterozostera tasmanica, Zostera muelleri and Ruppia megacarpa distribution and biomass in the Hopkins Estuary, western Victoria, by GIS. Aust J Bot 50:215-228
Jenkins G, Smith T (2008) Baywide Monitoring of Key Fishery Species in Seagrass Beds Sub-
Program. Milestone Report No. 1 (2008), September 2008. Technical Report No. 7,
Fisheries Victoria, Department of Primary Industries, Queenscliff, Victoria, Australia,
17pp.
Jenkins GP, Black KP, Hamer PA (2000) Determination of spawning areas and larval advection pathways for King George whiting in southeastern Australia using otolith microstructure and hydrodynamic modelling. I. Victoria. Mar Ecol-Prog Ser 199:231-242
Jenkins GP, Black KP, Wheatley MJ, Hatton DN (1997a) Temporal and spatial variability in recruitment of a temperate, seagrass-associated fish is largely determined by physical processes in the pre- and post-settlement phases. Mar Ecol-Prog Ser 148:23-35
Jenkins GP, Hamer PA (2001) Spatial variation in the use of seagrass and unvegetated habitats by post-settlement King George whiting (Percoidei: Sillaginidae) in relation to meiofaunal distribution and macrophyte structure. Mar Ecol-Prog Ser 224:219-229
Jenkins GP, Keough MJ, Hamer PA (1998) The contributions of habitat structure and larval supply to broad-scale recruitment variability in a temperate zone, seagrass-associated fish. J Exp
Mar Biol Ecol 226:259-278
Jenkins GP, May HMA, Wheatley MJ, Holloway MG (1997b) Comparison of fish assemblages associated with seagrass and adjacent unvegetated habitats of Port Phillip Bay and Corner
Inlet, Victoria, Australia, with emphasis on commercial species. Estuar Coast Shelf Sci
44:569-588
Jenkins GP, Sutherland CR (1997) The influence of habitat structure on nearshore fish assemblages in a southern Australian embayment: colonisation and turnover rate of fishes associated with artificial macrophyte beds of varying physical structure. J Exp Mar Biol
Ecol 218:103-125
Jenkins GP, Walker-Smith GK, Hamer PA (2002) Elements of habitat complexity that influence harpacticoid copepods associated with seagrass beds in a temperate bay. Oecologia
131:598-605
Jenkins GP, Wheatley MJ (1998) The influence of habitat structure on nearshore fish assemblages in a southern Australian embayment: Comparison of shallow seagrass, reef-algal and unvegetated sand habitats, with emphasis on their importance to recruitment. J Exp Mar
Biol Ecol 221:147-172
Jenkins GP, Wheatley MJ, Poore AGB (1996) Spatial variation in recruitment, growth, and feeding of postsettlement King George whiting, Sillaginodes punctata, associated with seagrass beds of Port Phillip Bay, Australia. Can J Fish Aquat Sci 53:350-359
Arthur Rylah Institute for Environmental Research 29
Review of Victorian Seagrass Research
Kendrick GA, Duarte CM, Marba N (2005) Clonality in seagrasses, emergent properties and seagrass landscapes. Mar Ecol-Prog Ser 290:291-296
Kendrick GA, Holmes KW, Van Niel KP (2008) Multi-scale spatial patterns of three seagrass species with different growth dynamics. Ecography 31:191-200
Kirkman H, Reid DD (1979) Study of the Role of the Seagrass Posidonia-Australis in the Carbon
Budget of an Estuary. Aquat Bot 7:173-183
Kuo J (2005) A revision of the genus Heterozostera (Zosteraceae). Aquat Bot 81:97-140
Lee KS, Park SR, Kim YK (2007) Effects of irradiance, temperature, and nutrients on growth dynamics of seagrasses: A review. J Exp Mar Biol Ecol 350:144-175
Leoni V, Vela A, Pasqualini V, Pergent-Martini C, Pergent G (2008) Effects of experimental reduction of light and nutrient enrichments (N and P) on seagrasses: a review. Aquat
Conserv-Mar Freshw Ecosyst 18:202-220
Macreadie PI, Hindell JS, Jenkins GP, Connolly RM, Keough MJ (2009) Fish Responses to
Experimental Fragmentation of Seagrass Habitat. Conserv Biol 23:644-652
Macreadie PI, Hindell JS, Keough MJ, Jenkins GP, Connolly RM ( in press ) Resource distribution determines positive edge effects in seagrass fish. Ecology
Marba N, Walker DI (1999) Growth, flowering, and population dynamics of temperate Western
Australian seagrasses. Mar Ecol-Prog Ser 184:105-118
Marguillier S, vanderVelde G, Dehairs F, Hemminga MA, Rajagopal S (1997) Trophic relationships in an interlinked mangrove-seagrass ecosystem as traced by delta C-13 and delta N-15. Mar Ecol-Prog Ser 151:115-121
Masini RJ, Manning CR (1997) The photosynthetic responses to irradiance and temperature of four meadow-forming seagrasses. Aquat Bot 58:21-36
Mateo MA, Cebrián J, Dunton K, Mutchler T (2006) Carbon flux in seagrass ecosystems. In:
Larkum WDA, Orth RJ, Duarte CM (eds) Seagrasses: Biology, Ecology and Conservation.
Springer, Dordrecht, The Netherlands, p 159-192
Meehan AJ, West RJ (2004) Seedling development and patch formation of the seagrass Posidonia australis in a southeast Australian estuary. Aquat Bot 79:1-14
Mellors J, Marsh H, Carruthers TJB, Waycott M (2002) Testing the sediment-trapping paradigm of seagrass: Do seagrasses influence nutrient status and sediment structure in tropical intertidal environments?, p 1215-1226
Melville AJ, Connolly RM (2005) Food webs supporting fish over subtropical mudflats are based on transported organic matter not in situ microalgae. Mar Biol 148:363-371
Moran SM, Jenkins GP, Keough MJ, Hindell JS (2003) Role of physical disturbance in structuring fish assemblages in seagrass beds in Port Phillip Bay, Australia. Mar Ecol-Prog Ser
251:127-139
Moran SM, Jenkins GP, Keough MJ, Hindell JS (2004a) Evidence for secondary planktonic transport of post-larvae of seagrass-associated King George whiting. Journal of Fish
Biology 64:1226-1241
Moran SM, Jenkins GP, Keough MJ, Hindell JS (2004b) Small-scale dynamics of secondary dispersal in a seagrass associated fish: a caging study. Mar Ecol-Prog Ser 272:271-280
Morris EP, Peralta G, Brun FG, van Duren L, Bouma TJ, Perez-Llorens JL (2008) Interaction between hydrodynamics and seagrass canopy structure: Spatially explicit effects on ammonium uptake rates. Limnology and Oceanography 53:1531-1539
Morris L, Jenkins G, Hatton D, Smith T (2007) Effects of nutrient additions on intertidal seagrass
(Zostera muelleri) habitat in Western Port, Victoria, Australia. Mar Freshw Res 58:666-
674
Murphy H (2007) Responses of meiofauna to edge effects, patch attributes and hydrodynamics.
B.Sc(Hons) thesis, University of Melbourne
Nagelkerken I, van der Velde G (2004a) Are Caribbean mangroves important feeding grounds for juvenile reef fish from adjacent seagrass beds? Mar Ecol-Prog Ser 274:143-151
Nagelkerken I, van der Velde G (2004b) Relative importance of interlinked mangroves and seagrass beds as feeding habitats for juvenile reef fish on a Caribbean island. Mar Ecol-
Prog Ser 274:153-159
30 Arthur Rylah Institute for Environmental Research
Review of Victorian Seagrass Research
Olsen JL, Stam WT, Coyer JA, Reusch TBH, Billingham M, Bostrom C, Calvert E, Christie H,
Granger S, La Lumiere R, Milchakova N, Oudot-Le Secq MP, Procaccini G, Sanjabi B,
Serrao E, Veldsink J, Widdicombe S, Wyllie-Echeverria S (2004) North Atlantic phylogeography and large-scale population differentiation of the seagrass Zostera marina
L. Mol Ecol 13:1923-1941
Orth RJ, Carruthers TJB, Dennison WC, Duarte CM, Fourqurean JW, Heck KL, Hughes AR,
Kendrick GA, Kenworthy WJ, Olyarnik S, Short FT, Waycott M, Williams SL (2006) A global crisis for seagrass ecosystems. Bioscience 56:987-996
Orth RJ, Marion SR, Granger S, Traber M (2009) Evaluation of a mechanical seed planter for transplanting Zostera malina (eelgrass) seeds. Aquat Bot 90:204-208
Paling EI, van Keulen M, Tunbridge DJ (2007) Seagrass transplanting in Cockburn Sound,
Western Australia: A comparison of manual transplantation methodology using Posidonia sinuosa Cambridge et Kuo. Restor Ecol 15:240-249
Parry GD (2007) Feasibility of restoration of seagrass beds using seeds. Marine and Freshwater
Systems Report No. 21, Primary Industries Research Victoria, Queenscliff. Report No. 21
Parry GD, Heislers S, Chan K, Cleaver J, Werner GF (2005) Seasonality of flowering and fruiting of the seagrasses Zostera mulleri and Zostera (formerly Heterozostera ) tasmanica ,
Primary Industries Research Victoria, Queenscliff
Procaccini G, Olsen JL, Reusch TBH (2007) Contribution of genetics and genomics to seagrass biology and conservation. J Exp Mar Biol Ecol 350:234-259
Procaccini G, Piazzi L (2001) Genetic polymorphism and transplantation success in the mediterranean seagrass Posidonia oceanica. Restor Ecol 9:332-338
Rattray A, Ierodiaconou D, Laurenson L, Burq S, Reston M (2009) Hydro-acoustic remote sensing of benthic biological communities on the shallow South East Australian continental shelf.
Estuar Coast Shelf Sci 84:237-245
Roob R, Ball D (1997) Victorian Marine Habitat Database: Gippsland Lakes Seagrass Mapping.
Marine and Freshwater Resources Institute. (Marine and Freshwater Resources Institute:
Queenscliff), November 1997
Roob R, Morris P, Werner G (1998) Victorian Marine Habitat Database: Corner Inlet/Nooramunga
Seagrass Mapping. Marine and Freshwater Resources Institute. (Marine and Freshwater
Resources Institute: Queenscliff), July 1998
Shepherd SA, Watson JE, Womersley HBS, Carey JM (2009) Long-term changes in macroalgal assemblages after increased sedimentation and turbidity in Western Port, Victoria,
Australia. Botanica Marina 52:195-206
Sintes T, Marba N, Duarte CM, Kendrick GA (2005) Nonlinear processes in seagrass colonisation explained by simple clonal growth rules. Oikos 108:165-175
Smith J, Macreadie PI, Swearer SS ( in press-a ) An osmotic induction method for externally marking a saltwater fish (Stigmatopora pipefish) with calcein. Journal of Fish Biology
Smith TM, Hindell JS, Jenkins GP, Connolly RM (2008) Edge effects on fish associated with seagrass and sand patches. Mar Ecol-Prog Ser 359:203-213
Smith TM, Hindell JS, Jenkins GP, Connolly RM ( in press-b ) Seagrass patch size affects fish responses to edges. Journal of Animal Ecology
Spruzen FL, Richardson AMM, Woehler EJ (2008) Influence of environmental and prey variables on low tide shorebird habitat use within the Robbins Passage wetlands, Northwest
Tasmania. Estuar Coast Shelf Sci 78:122-134
Torquemada YF, Durako MJ, Lizaso JLS (2005) Effects of salinity and possible interactions with temperature and pH on growth and photosynthesis of Halophila johnsonii Eiseman. Mar
Biol 148:251-260
Touchette BW (2007) Seagrass-salinity interactions: Physiological mechanisms used by submersed marine angiosperms for a life at sea. J Exp Mar Biol Ecol 350:194-215
Touchette BW, Burkholder JM (2000) Review of nitrogen and phosphorus metabolism in seagrasses. J Exp Mar Biol Ecol 250:133-167
Valentine JF, Duffy JE (2006) The central role of grazing in seagrass ecology. In: Larkum WDA,
Orth RJ, Duarte CM (eds) Seagrasses: Biology, Ecology and Conservation. Springer,
Dordrecht, The Netherlands, p 463-501
Arthur Rylah Institute for Environmental Research 31
Review of Victorian Seagrass Research van Katwijk MM, Bos AR, de Jonge VN, Hanssen L, Hermus DCR, de Jong DJ (2009) Guidelines for seagrass restoration: Importance of habitat selection and donor population, spreading of risks, and ecosystem engineering effects. Marine Pollution Bulletin 58:179-188
Verduin JJ, Backhaus JO (2000) Dynamics of plant-flow interactions for the seagrass Amphibolis antarctica: Field observations and model simulations. Estuar Coast Shelf Sci 50:185-204
Vermaat JE (2009) Linking clonal growth patterns and ecophysiology allows the prediction of meadow-scale dynamics of seagrass beds. Perspect Plant Ecol Evol Syst 11:137-155
Walker D, Dennison W, Edgar G (1999) Status of Australian seagrass research and knowledge. In:
Butler A, Jernakoff P (eds) Seagrass in Australia. CSIRO Publishing, Collingwood,
Victoria, p 1-18
Walker DI (1985) Correlations between Salinity and Growth of the Seagrass Amphibolis-
Antarctica (Labill) Sonder and Aschers in Shark Bay, Western-Australia, Using a New
Method for Measuring Production-Rate. Aquat Bot 23:13-26
Walker DI, McComb AJ (1990) Salinity Response of the Seagrass Amphibolis-Antarctica (Labill)
Sonder Et Aschers - an Experimental Validation of Field Results. Aquat Bot 36:359-366
Warry FY, Hindell JS, Macreadie PI, Jenkins GP, Connolly RM (2009) Integrating edge effects into studies of habitat fragmentation: a test using meiofauna in seagrass. Oecologia
159:883-892
Waycott M (1998) Genetic variation, its assessment and implications to the conservation of seagrasses. Mol Ecol 7:793-800
Waycott M, Duarte CM, Carruthers TJB, Orth RJ, Dennison WC, Olyarnik S, Calladine A,
Fourqurean JW, Heck KL, Hughes AR, Kendrick GA, Kenworthy WJ, Short FT, Williams
SL (2009) Accelerating loss of seagrasses across the globe threatens coastal ecosystems.
Proc Natl Acad Sci U S A 106:12377-12381
Waycott M, James SH, Walker DI (1997) Genetic variation within and between populations of
Posidonia australis, a hydrophilous, clonal seagrass. Heredity 79:408-417
Waycott M, Walker DI, James SH (1996) Genetic uniformity in Amphibolis antarctica, a dioecious seagrass. Heredity 76:578-585
Winning MA, Connolly RM, Loneragan NR, Bunn SE (1999) N-15 enrichment as a method of separating the isotopic signatures of seagrass and its epiphytes for food web analysis. Mar
Ecol-Prog Ser 189:289-294
32 Arthur Rylah Institute for Environmental Research
Review of Victorian Seagrass Research
Arthur Rylah Institute for Environmental Research 33
ISSN 1835-3827 (print)
ISSN 1835-3835 (online)
ISBN XXXXXXXX (print)
ISBN XXXXXXXX (online)