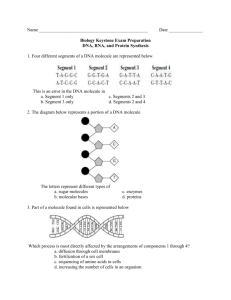
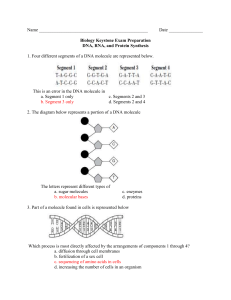
VI. Nucleic Acids and Protein Synthesis
advertisement

Shier, Butler, and Lewis: Hole’s Human Anatomy and Physiology, 12th ed. Chapter 4: Cellular Metabolism Chapter 4: Cellular Metabolism I. Introduction and II. Metabolic Processes A. Introduction 1. Metabolism is the sum total of chemical reactions within cells. 2. In metabolic reactions, the product of one reaction serves as starting materials for another metabolic reaction. 3. This chapter explores how metabolic pathways supply energy to a cell and how other biochemical processes enable a cell to produce proteins. 4. Two types of metabolic reactions and pathways are anabolism and catabolism. 5. In anabolism, larger molecules are constructed from smaller ones. 6. In catabolism, larger molecules are broken down into smaller ones. 7. Anabolism requires energy. 8. Catabolism releases energy. B. Anabolism 1. Anabolism provides all the materials required for cellular growth and repair. 2. Dehydration synthesis joins many simple sugar molecules to form larger molecules of glycogen. 3. When monosaccharides are joined, they form glycogen and a hydroxyl group from one monosaccharide and a hydrogen atom from another monosaccharide are removed. 4. The H and OH react to produce water. 5. Glycerol and fatty acid molecules join by dehydration synthesis to form fat molecules. 6. The result is three water molecules and one fat molecule. 7. Dehydration synthesis also builds proteins by joining amino acids. 8. The type of bond that holds amino acids together is a peptide bond. 9. A polypeptide is a chain of amino acids. C. Catabolism 1. Catabolism is a group of metabolic processes that break down larger molecules into smaller ones. 2. An example of catabolism is hydrolysis, which can decompose carbohydrates, lipids, and proteins. 3. In hydrolysis, a water molecule is used to split substances. 4. The hydrolysis of a disaccharide results in two monosaccharides. 5. When the bond between simple sugars breaks, water supplies a hydrogen atom to one sugar molecule and a hydroxyl group to the other. 6. Hydrolysis is the reverse of dehydration synthesis. 7. Hydrolysis breaks down carbohydrates into monosaccharides. 8. Hydrolysis breaks down fats into glycerol and fatty acids. 9. Hydrolysis breaks down proteins into amino acids. 10. Hydrolysis breaks down nucleic acids into nucleotides. III. Control of Metabolic Reactions A. Enzyme Action 1. Metabolic reactions require energy before they proceed. 2. Heat energy increases the rate at which molecules move and the frequency of molecular collisions. 3. The collisions of particles increase the likelihood of interactions among the electrons of the molecules that can form new chemical bonds. 4. Most enzymes are globular proteins that promote specific chemical reactions in cells by lowering the activation energy required to start these chemical reactions. 5. Enzymes are needed in very small quantities because as they work, they are not consumed and can function repeatedly. 6. Each enzyme is specific, acting only on a particular molecule, which is called its substrate. 7. A substrate is a substance on which an enzymes acts. 8. The substrate of catalane is hydrogen peroxide. 9. The ability of an enzyme to identify a substrate depends on the shape of its active site. 10. Active sites are regions of enzymes that bind specifically to substrates. 11. The interaction of the enzyme-substrate complex causes chemical bonds to be strained in a substrate in a way that makes a chemical reaction more likely to occur. 12. The speed of enzyme-catalyzed reactions depends on the number of enzymes and substrate molecules. 13. Metabolic pathways are sequences of enzyme-controlled reactions. 14. Enzyme names are often derived from the names of their substrates with the suffix –as added. B. Regulation of Metabolic Pathways 1. The rate at which a metabolic pathway functions is often determined by a regulatory enzyme. 2. The number of molecules of a regulatory enzyme is limited. 3. The product of a metabolic pathway may inhibit the rate-limiting enzyme. 4. When the product inhibits the enzyme, this is an example of negative feedback. C. Cofactors and Coenzymes 1. A cofactor helps an active site obtain its appropriate shape or helps bind the enzyme to its substrate. 2. Examples of cofactors include copper, iron, or zinc. 3. Coenzymes are organic molecules that act as cofactors. 4. Examples of coenzymes are vitamins. 5. Vitamins are essential organic molecules that human cells cannot synthesize. D. Factors That Alter Enzymes 1. Almost all enzymes are proteins. 2. Five factors that can denature enzymes are heat, radiation, certain chemicals, electricity, and changes in pH. IV. Energy for Metabolic Reactions A. Introduction 1. Energy is the capacity to change something; it is the ability to do work. 2. Six forms of energy are heat, light, sound, electricity, mechanical energy, and chemical energy. 3. Energy can be changed from one form to another form. 4. All metabolic reactions involve energy in some form. B. ATP Molecules 1. The three main parts of an ATP molecule are an adenine, a ribose, and three phosphates in a chain. 2. The third phosphate of ATP is attached by a high-energy bond. 3. When the terminal phosphate bond of ATP is broken, energy is released. 4. Energy from the breakdown of ATP powers cellular work such as skeletal muscle contraction, active transport across cell membranes, and secretion. 5. An ATP molecule that loses its terminal phosphate becomes ADP. 6. ADP has two phosphates. 7. ATP can be resynthesized from an ADP by the process called phosphorylation. 8. Without enough ATP, cells quickly die. C. Release of Chemical Energy 1. Most metabolic processes depend on chemical energy stored in ATP. 2. Chemical energy is initially held in chemical bonds that link atoms into molecules. 3. Chemical energy is released when energy-containing chemical bonds break. 4. Burning a marshmallow over a fire releases chemical energy as heat and light. 5. Cells “burn” glucose molecules in a process called oxidation. 6. The energy released by oxidation of glucose is used to promote cellular metabolism. 7. In cells, enzymes initiate oxidation by lowering the activation energy. 8. Cellular respiration is the process that releases energy from molecules such as glucose and makes it available for cellular use. V. Cellular Respiration A. Introduction 1. The three series of reactions of cellular respiration are glycolysis, citric acid cycle, and electron transport chain. 2. The products of cellular respiration are carbon dioxide, water, and energy. 3. In cellular respiration some energy is lost as heat but almost half is captured as ATP. 4. Aerobic reactions are different from anaerobic reactions in that they require oxygen. 5. For each glucose molecule that is decomposed by cellular respiration, up to 38 ATP molecules are produced. 6. All but two ATPs are formed by the aerobic reactions. B. Glycolysis 1. Glycolysis is a series of ten enzyme-catalyzed reactions that break down the 6-carbon glucose molecule into two 3-carbon pyruvic acid molecules. 2. Glycolysis occurs in the cytoplasm. 3. Glycolysis is referred to as the anaerobic phase of respiration. 4. In the first main event of glycolysis, glucose is phosphorylated by the addition of two phosphates. 5. The first main event of glycolysis requires ATP. 6. In the second main event of glycolysis, glucose is split into two 3-carbon molecules. 7. In the third main event of glycolysis, the electron carrier NADH is produced, ATP is synthesized and two pyruvic acid molecules result. 8. NADH delivers high-energy electrons to the electron transport chain where ATP is produced. D. Anaerobic Reactions 1. Oxygen acts as the final electron acceptor at the end of the electron transport chain. 2. Under anaerobic conditions, the electron transport chain has nowhere to unload its electrons. 3. Under anaerobic conditions, pyruvic acid forms lactic acid. 4. The buildup of lactic acid inhibits glycolysis. E. Aerobic Reactions 1. If oxygen is available, pyruvic acid can continue through the aerobic pathways. 2. The reactions of the aerobic pathways are synthesis of acetyl CoA, the citric acid cycle, and the electron transport chain. 3. Three products of the aerobic pathways are carbon dioxide, water, and ATP. 4. For each pyruvic acid, enzymes in the mitochondria are used to generate three products, NADH, carbon dioxide, and acetic acid. 5. Acetic acid combines with coenzyme A to form acetyl CoA. 6. The citric acid cycle begins when acetyl CoA combines with oxaloacetic acid to form citric acid. 7. In the cycle, citric acid is changed through a series of chemical reactions back into oxaloacetic acid. 8. The cycle repeats as long as the mitochondrion receives oxygen and pyruvic acid. 9. Three important consequences of the citric acid cycle are one ATP is produced directly for each citric acid molecule that goes through the cycle, eight hydrogen atoms with high-energy electrons are transferred to the hydrogen carriers NAD+ and FAD, and two carbon dioxide molecules are produced. 10. The electron transport chain is a series of enzyme complexes that carry and pass electrons along from one to another. 11. The electron transport chain is located in the inner mitochondrial membrane. 12. The chain lowers energy levels of electrons and transfers energy to ATP synthase. 13. ATP synthase uses energy to phosphorylate ADP into ATP. 14. At the end of the chain, hydrogen atoms and oxygen combine to form water. 15. Excess glucose in cells may enter anabolic pathways and be linked into storage forms such as glycogen. 16. When blood glucose levels are high, the liver uses glucose to synthesize glycogen. 17. When blood glucose levels are low, the liver releases glucose. 18. When a person takes in more carbohydrates than can be stored as glycogen, glucose is used to form fat molecules. VI. Nucleic Acids and Protein Synthesis A. Introduction 1. The information that instructs a cell to synthesize specific proteins is held in DNA. 2. The genetic code is the correspondence between a unit of DNA information and a particular amino acid. B. Genetic Information 1. Chromosomes are long molecules of DNA and associated proteins. 2. A gene is a portion of a DNA molecule that contains the genetic information for making a particular protein. 3. All four groups of organic molecules require genetic instructions because enzymes control their synthesis. 4. A genome is the complete set of genetic instructions in a cell. 5. Nucleotides are building blocks of nucleic acids. 6. Three parts of a nucleotide are a sugar, a phosphate group, and one of several organic, nitrogen-containing bases. 7. A DNA molecule consists of two polynucleotide chains. 8. In DNA, bases of the first strand bind by hydrogen bonds to bases of the second strand. 9. The four bases found in DNA are adenine, thymine, cytosine, and guanine. 10. In DNA, adenine always binds with the base thymine. 11. In DNA, guanine always binds with the base cytosine. 12. If the sequence of bases of one strand of DNA is GACT the bases of the complementary strand of DNA are CTGA. 13. DNA twists to form a helix. 14. In the nucleus, DNA is wound around histones. C. DNA Replication 1. DNA replication is the process that creates an exact copy of a DNA molecule. 2. DNA replication occurs during interphase of the cell cycle. 3. As replication begins, hydrogen bonds break between the complementary base pairs of the double strands comprising the DNA molecule. 4. New nucleotides pair with the exposed bases. 5. DNA polymerase catalyzes the base pairing. 6. Each new DNA molecule is composed of one old strand and one new strand. D. Genetic Code 1. Genetic information specifies the correct sequence of amino acids in a polypepetide chain. 2. Each amino acid is represented in a DNA molecule by a triplet code. 3. A triplet code consists of a sequence of three nucleotides. 4. The genetic code is the method of storing information for protein synthesis. 5. RNA molecules function to transfer information from the nucleus to the cytoplasm. E. RNA Molecules 1. The sugar in RNA is ribose. 2. RNA is single stranded. 3. The four bases found in RNA are adenine, uracil, guanine, and cytosine. 4. In the synthesis of mRNA, RNA nucleotides form complementary base pairs with a section of DNA. 5. The enzyme RNA polymerase controls mRNA synthesis. 6. In mRNA synthesis, if the sequence of DNA bases is TACCCGAGG, the complementary bases in the developing mRNA are AUGGGCUCC. 7. Synthesis of mRNA stops when RNA polymerase reaches a termination signal on DNA. 8. Transcription is the making of mRNA from DNA. 9. Codons are three base sequences on mRNA. 10. To complete protein synthesis, mRNA leaves the nucleus and associates with a ribosome. 11. Translation is the process in which the series of codons on mRNA are translated from the language of nucleic acids to the language of amino acids. F. Protein Synthesis 1. Transfer RNA functions to align amino acids in a way that enables them to bond. 2. One end of a tRNA molecule contains an anticodon and the other end contains an amino acid. 3. An anticodon is a three base sequence on tRNA. 4. The nucleotides of the anticodon bind to nucleotides of the codon. 5. There are twenty types of amino acids. 6. There are sixty-four codons possible. 7. Three codons provide a stop signal. 8. A stop signal indicates the end of protein synthesis. 9. More than one type of tRNA can correspond to the same amino acid. 10. The genetic code is degenerate because a given amino acid can be specified by more than one codon. 11. A ribosome is composed of two subunits that contain rRNA and proteins. 12. The smaller subunit functions to bind to a molecule of mRNA near the codon at the beginning of the messenger strand. 13. The larger subunit functions to hold a growing chain of amino acids, and provides some enzymes necessary for the bonding of amino acids. 14. Chaperones function to fold proteins into their unique shapes. 15. The number of protein molecules a cell synthesizes is usually proportional to the number of corresponding mRNA molecules. 16. Transcription factors control the activation of certain genes. VII. Changes in Genetic Information A. Introduction 1. A mutation is an alteration of genetic material. 2. Some mutations can cause devastating medical conditions. B. Nature of Mutations 1. Five ways mutations can occur are through incorrect base pairings during DNA replication, adding extra bases in DNA, deleting sections of DNA, moving sections of DNA within the same chromosome, or moving sections of DNA from one chromosome to another. 2. Mutations may cause proteins to be abnormal and nonfunctional. 3. Repair enzymes are enzymes that clip out mismatched nucleotide sequences in a single DNA strand and fill the resulting gap with nucleotides complementary to those on the other strand. C. Effects of Mutations 1. Usually two or three codons specifying the same amino acid differ only in the third base of the codon. 2. A mutation that changes the third codon base can encode the same amino acid. 3. If a mutation alters a base in the second position, the protein is usually not significantly changed. 4. Another protection against mutation is that a person has two copies of each chromosome and therefore each gene. 5. Mutagens are factors that cause mutations. 6. An inborn error of metabolism results from inheriting a mutation that alters an enzyme.