CH 3 ppt guided rdg
advertisement

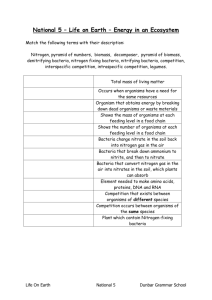
ENERGY FLOW AND MATTER CYCLES CHAPTER 3 NAME _____________________________________ DATE _________________ PERIOD _______ WHERE DO MOST ORGANISMS GET THEIR ENERGY? The ___________! Plants, algae and some kinds of bacteria capture the sun light and produce ________________, an energy rich food. This process is called ________________________________________. 6_________ + 6_________+ _________ → C6H12O6 + 6___________ C6H12O6 is the _____________________________ which supplies the _______________ when bonds are broken during respiration. ___________________________ (autotroph)- an organism that gets its energy from the sun. Examples are grass, clover, trees ___________________________ (heterotroph)- organisms that eat other organisms. Examples are rabbits, lions and spiders Consumers get their energy indirectly from the ___________ by eating producers or other organisms that eat producers. _____________________________ eat only producers such as cows, sheep, deer and grasshoppers _____________________________ eat only other consumers such as hawks and lions Omnivores- eat both plants and animals such as humans, pigs and bears Scavengers- eat remains of dead animals such as vultures and _______________. Decomposers- get their food by breaking down dead organisms such as _____________________ and __________________ WHAT IS CELLULAR RESPIRATION? ________________________ C6H12O6+ 6O2 → 6CO2 +6H20 + energy It is the process of breaking down food to get Is an aerobic process ENERGY TRANSFER • Each time an organism eats another, a transfer of ___________________ occurs. • We use food chains, food webs and trophic levels to follow the flow of food energy. • Trophic= feeding level • A simple representation of how energy is transferred. Food _____________ • A food _______ is a more accurate representation of what is really happening when organisms eat. ENERGY PYRAMIDS • Each layer is a _____________________ (feeding) layer Producers form the base level. • Heterotrophs make up the second level and carnivores are on the rest of the levels • As you go up the side of the pyramid, the amount of energy __________________________ and the number of organisms also _____________________________. HOW DOES ENERGY LOSS AFFECT AN ECOSYSTEM? 1. There are _____________ organisms at the higher trophic levels. 2. The loss of energy to each trophic level ___________________the number of levels in an ecosystem. 3. There are usually no more than 4-5 levels because there is not enough __________________ left to support higher trophic levels. This is an energy pyramid. Notice there is a ______% loss of energy as you go up the pyramid. What do you notice about the number of organisms present at each trophic level? 3.2 RESOURCE RECYCLING IN NATURE: THE BIOGEOCHEMICAL CYCLES There are 4 major cycles: • Hydrologic (water) Mineral/nutrient ___________________ Nitrogen THE NUTRIENT/MINERAL CYCLE • Includes all the nutrients needed by organisms. • Minerals originate in ____________and are released when rocks _____________________. • Eventually these minerals end up in the _____________________ and soil. THE CARBON CYCLE • A key element in _______________________ molecules. • Plants take in CO2 during photosynthesis and all organisms release it during _________________________ (returned to the air). • Stored as a _________________________ (C6H12O6) • Returned to air when organisms die and decompose. • Can be stored for long periods of time in ____________________, ____________________, shells and skeletons of marine organisms (CaCO3) • Carbon is __________________ in fossil fuels and released when they ____________________. • Fossil fuels = oil, coal, natural gas, ____________________________ and ______________________. • CO2 is a __________________________________ and is believed to contribute to global warming. • Carbon sinks= ocean and _________________ THE NITROGEN CYCLE Organisms need nitrogen to make _______________________. Nitrogen gas (N2) makes up _________% of the atmosphere. Plants need nitrogen to _____________, but cannot use it directly from the atmosphere. Nitrogen-fixing ______________________ convert the N2 into a useable form. This is called nitrogen fixation. Some plants have nitrogen-fixing bacteria in __________________ on their roots which house the bacteria- a mutualistic relationship. These plants are legumes such as peas, soy beans and alfalfa. Other bacteria live in the _______________ and add the nitrogen to the soil. How does nitrogen get “fixed”? Atmospheric nitrogen is converted by ________________________ into ammonia (NH3). Ammonia is then converted into nitrite and then nitrate by nitrifying bacteria. Plants then take in the fixed nitrogen. Animals get their needed nitrogen by ______________________________________. Not a lot of nitrogen in the soil, so it is added to the soil as animal manure and fertilizer. 3.3 MATTER AND ENERGY 3.4 KINETIC AND POTENTIAL ENERGY • Mass, matter and energy • Potential and kinetic energy GIVE EXAMPLES OF POTENTIAL AND KINETIC ENERGY 3.5 CLOSED AND OPEN SYSTEMS • All cycling occurs in a ______________________. • A system is a designated area, space or region under study. • Earth is a ____________________ system as far as matter goes. • Earth is an __________________ system as far as energy goes. • Closed system: nothing enters or leaves. Everything is ____________________________________. • Open system: things both enter and leave. • Steady state: properties are constant because substances enter and leave at the ____________ _____________. • Using figure 3.15, answer question 1 on page 85. 3.6 CONSERVATION LAWS • Law Of Conservation Of Matter – total mass on earth is _______________________. • Law of Conservation of Energy- energy cannot be created or destroyed. It can, however, be _______________________from one form to another ( mechanical → heat i.e. rubbing hands together ). • This law is also known as the First Law Of Thermodynamics. WHY ARE THESE LAWS IMPORTANT IN ENVIRONMENTAL SCIENCE? 6 FORMS OF ENERGY • Mechanical • Electrical Heat (thermal) Radiant (including light) ___________________ ____________________ ENERGY SOURCES • The Sun – Energy drives the __________________, waves, and climate. – Provides energy for photosynthesis. – Fuels all life processes! – Only _______% of sun’s radiation reaches Earth, and only ________% of that reaches the atmosphere and surface. Solar energy is stored in living __________________. These plants can form oil, coal, natural gas, oil shale, tar sands • Tides • Earth’s Heat – Geothermal energy • Fission Fuels – Energy stored in unstable uranium and thorium _______________ – Nuclear energy • Fusion Fuels – Involves combining small nuclei into larger nuclei. By combining nuclei (deuterium and tritium), energy is released. (the sun) 3 PARTS TO THE 2ND LAW OF THERMODYNAMICS • 1. In any transformation of energy from one form to another, there is always a __________________ in the amount of useful energy. • 2. Heat cannot, by itself, flow from cold to hot. It spontaneously flows from ____________________. • http://www.youtube.com/watch?feature=fvwrel&v=C0NBosKaznA&NR=1 • 3. In any closed system, disorder has a natural tendency to _______________________. (chaos, randomness, entropy) THE KINETIC THEORY OF MATTER: THE KINETIC THEORY IS BASED ON 3 ASSUMPTIONS. • 1. All matter is made of ______________________________ or ions. • 2. Atoms, molecules and ions are in constant _________________. The greater the motion of the particles, the warmer the object. • 3. Moving particles do not lose energy when they collide with each other or with a rigid container. • Efficiency = useful energy or work x 100% energy or work in OR Efficiency = work output work input x 100% No efficiency can be greater than ________% • You can’t get ___________work out of any process than what you put in. • Energy is often lost to _________________ (heat) so industry tries to reduce friction by using lubricants, making machines smooth…. Heat engines and lighting are very inefficient. • See figures 3.39 and 3.40 • Study figures 3.41 and 3.42 • Complete the Questions/Tasks on pages 112 and 113 of your text. • Net Energy - _______________ of total energy produced over the lifetime of the system to the total energy, direct and indirect, used to produce that energy. • Energy Quality – how ___________________ is the energy? ___________________________ energy can be used to move things or generate electricity. It is organized and concentrated. __________________ temperature heat is low quality. It is heat at a temperature near that of the surroundings. It cannot be used to produce mechanical or electrical energy. DEVELOPED COUNTRIES ARE ENERGY DEPENDENT • Therefore, energy quality is important. • Most activities result in matter becoming more ___________________________ and as energy is transformed, it goes to ___________________________ forms. Our energy supply is continuously losing its ability to move objects and produce electricity. • What can be done to improve our use of energy? • http://www.youtube.com/watch?v=HOQSAjc37Y