Besnoitia besnoiti impact on fertility of cattle exploited in
advertisement

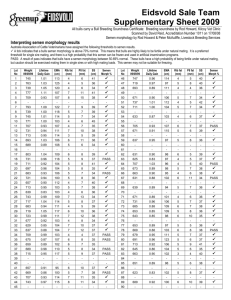
Besnoitia besnoiti impact on fertility of cattle exploited in Mediterranean pastures (Alentejo). H. Cortes1, Chagas e Silva, J.2, Baptista, M.C. 3, Pereira, R.M. 3, Horta, A.E.M.3, Vasques, M.I. 3, Barbas, J.P. 3, Marques, C.C3, Leitão, A.4, 1 Laboratório de Parasitologia. Núcleo da Mitra. Universidade de Évora (ICAM), Portugal. 2 Divisão de Selecção e Reprodução Animal, DGV, Venda Nova, Amadora, Portugal 3 Departamento de Reprodução Animal, Estação Zootécnica Nacional, Santarém, Portugal 4 Instituto de Investigação Científica Tropical (CIISA), Lisboa, Portugal. Abstract Besnoitia besnoiti is a bovine parasite endemic in many tropical and subtropical areas whose prevalence in the Mediterranean countries such as Portugal seems to be increasing. Most infections are mild or subclinical, characterized by the formation of large numbers of cutaneous and sub-cutaneous cysts, lowering the quality of skins for leather industry. Male sterility or impaired fertility is a common sequela in breeding bulls, and is one of the most negative aspects of the disease in animals that survive the acute and chronic stages of infection. Our objective was to investigate if asymptomatic besnoitiosis leads to bovine infertility, by comparing seminal parameters pre and postthawing and in vitro fertilization and embryo development rates between asymptomatic infected (n=3) and uninfected (n=5) bulls, under an extensive production system in Alentejo-Portugal. Skin biopsies were collected and submitted to histopathological analyses to identify the presence of B. besnoiti cysts in sires. Semen was collected by electroejaculation and data of sperm quality parameters before and after cryopreservation were analyzed using ANOVA. The quality of semen collected from asymptomatic infected and uninfected bulls presented no differences before cryopreservation. From all the sperm post-thawed quality parameters evaluated (motility and hypoosmotic swelling tests; motility, activity, concentration and agglutination post-swim-up; fertilization and embryo development rates) only postthawed (51.0±36.3 vs 42.3±10.6%, P=0.05) and post-swim-up (36.3±18.8 vs 25.1±12.0, P=0,009) motility were significantly different between asymptomatic infected and uninfected bulls, respectively. Semen from asymptomatic Besnoitia infected bull may maintain fertilization ability although the presence of these animals in herds implies a potential transmission of the disease leading to further economic losses. Keywords: Besnoitia besnoiti, Bovine besnoitiosis, infertility, Portugal. Introduction Besnoitia besnoiti (Marotel 1912) is a coccidian parasite of cattle that is closely related to Toxoplasma gondii. B. besnoiti is endemic in many tropical and subtropical areas where it causes both acute and chronic bovine besnoitiosis, leading to significant economic constraint to commercial cattle production (Bigalke & Schoeman, 1967, Shkap et al., 1994). In Europe, it has been reported only in the Mediterranean countries, France (Besnoit & Robin, 1912, Bourdeau et al., 2004) Spain (Juste et al., 1990, Irigoien et al., 2000), and Portugal. In the latter country, prior to 1991 (Malta & Silva, 1991), the disease was seldom recognized or reported (Franco & Borges, 1915, Leitão, 1949). At present its prevalence may be increasing (Cortes et al., 2003, Cortes et al., 2005). All breeds, both sexes, and animals of all ages are affected by this parasitosis, although clinical disease occurs rarely in calves less than 6 months of age (Bigalke, 1968). The occurrence is usually sporadic, and only a small proportion of infected animals develop clinical disease (Pols, 1960, Bigalke, 1968). In the acute stage of disease, parasitic tachyzoites invade blood vessels of the skin, subcutaneous tissues, fascia and testes causing widespread vasculitis and thrombosis. The result is a severe generalized reaction which is accompanied by oedema of the skin and acute orchitis. During the chronic stage, large numbers of tissue microcysts containing bradyzoites are formed in the skin (fig. 1). The most striking features of this stage are thickening, wrinkling and hair loss of the skin (fig. 2), accompanied by anorexia and severe weight loss. The case fatality rate is approximately 10% (McCully et al., 1966, Basson et al., 1970). Irreversible male infertility or impaired fertility is a common sequela in breeding bulls, and is one of the most negative aspects of the disease in animals that survive the acute and chronic stages of infection (Basson et al., 1970, Kumi-Diaka et al., 1981, Ferreira et al., 1982, Cortes, et al., 2005). The main objective of this experimental work was to compare fertility parameters between uninfected and infected bulls where at histopathology, cysts where identified in the skin, without showing any other clinical signs of disease and, within predict some implications of their presence on farm reproduction. Material and Methods Animals. Farms with historical cases of bovine besnoitiosis were chosen for this study. All the farms were located in Évora region (Portugal) and exploit beef cattle extensively. Eight animals, seven Limousine and one Charolais bull, aged 2-9 years, were selected in 3 different farms from this region. Skin samples were collected from the neck of several bulls and fixed in 10% formalin solution, to identify the presence of Besnoitia cyst in it. Skin samples were stained with Hematoxilin/Eosin and the presence of cysts was searched with 100X amplification in a light microscope. The target was finding males without any sign of disease, but histopathology revealing the presence of Besnoitia cysts. This was achieved in three bulls. Semen collection and cryopreservation. Semen was collected by electroejaculation to asymptomatic infected (n=3) and uninfected bulls (n=5) and immediately stored in a 32ºC water bath. Before cryopreservation, semen morphology was evaluated by determination of gross (1 to 5) and individual progressive (0 – 100%) motility, percentage of abnormal and of live (supravital stain eosin–nigrosin) spermatozoa (spz). After sperm concentration determination using a single Neubauer hemacytometer, dilutions were performed using Triladyl (ref. 13500/0250, Minitub GmbH) with egg yolk and distilled water, previously warmed at 32ºC, to obtain progressively motile 20x106 spermatozoa per seminal dose. Extended semen was immediately loaded into 0.25 ml straws. All the straws were vertically stored in appropriate device and maintained at 5ºC for 4 hours and then frozen by vertical freezing method (Jondet, 1972 modified by Chagas e Silva, 1992). The rackets with straws were kept inside the RCB4 Container (Air Liquide, France) in the nitrogen vapours (on the position that, over a 4 cm of liquid nitrogen, the devise touches the liquid phase) for 20 minutes with the lid closed, before being placed into appropriate cryogenic containers and immersed into liquid nitrogen (-196ºC) for storage. Post-thawed semen evaluation Post-thawed semen samples from all ejaculates were subjected to evaluation of motility, hypoosmotic swelling, post-swim-up tests, in vitro fertilization, and embryo development. The effect of B. Besnoiti on these parameters was investigated according to the following experimental design: In experiment 1 (6 replicates), semen from uninfected bulls (n=5) was tested. In experiment 2 (5 replicates) semen was used from two uninfected bulls, with the worst and the best fertilizing ability selected in experiment 1, and from three asymptomatic infected bulls. For the hypoosmotic swelling test only two straws from each infected and uninfected animals were evaluated. Post-thawed motility and Host Individually progressive semen motility after thawing from infected or uninfected sires was evaluated. To perform the host test two straws from each bull were thawed. The hypoosmotic solution consisted of tri-sodium citrate (10.4g L-1) with100 mOsm L-1 of final osmolarity. Aliquots (100 µl) of semen were incubated with 1 ml of the hypoosmotic solution for 5, 25 and 40 minutes. After each incubation period, a droplet of this mixture was pippeted on to a slide and fixed with 2% glutaraldeyde in BL-1 medium (Pursel et al., 1972) and 100 spermatozoa were examined in each preparation. Post-swim-up tests To perform the post-swim-up tests semen was thawed and incubated for 1 hour in TALP medium without calcium or antibiotics supplemented with 48,55 l mL-1 caffeine (Sigma, ref. C-0750) in an humidified atmosphere of 5% CO2 at 39ºC. Spermatozoa from the upper layer were taken to evaluate their individual motility (0 – 100%), vitality (1 to 5), concentration and agglutination (number of agglutinated heads in 100 observed spermatozoa) post-swim-up. In vitro fertilization and embryo development In vitro maturation, fertilization and co-culture procedures have been described previously (Pereira et al., 2005). Briefly, follicles with 2-6 mm diameter were aspirated from slaughterhouse bovine ovaries. Selected cumulus oocyte complexes were matured in TCM199 (GibCo, ref.22340-020) with 10% superovulated oestrus cow serum (SOCS), 10 µg mL-1 FSH (Follotropin, vetrepham Inc.) and antibiotics (Sigma, ref. P0781) during 22-24 hours. For fertilization, frozen-thawed semen was submitted to swim-up as above. Spermatozoa (106 spz mL-1) and 10 oocytes were placed in 40 µL droplets of TALP medium supplemented with 5.4 USP mL-1 heparin (Sigma, ref. H3393), 10 mM penicillamine (Sigma, ref. P-4875), 20 mM hypotaurine (Sigma, ref. H- 1384) and 0.25 mM epinephrine (Sigma, ref. E-1635). Following co-incubation for 22 hours the presumptive zygotes were randomly placed in 100 µL droplets of a granulosa cell monolayer cultured with TCM199, 10% SOCS and antibiotics under paraffin oil, where embryo culture proceeded. Atmospherical conditions for IVM-IVF and embryo co-culture were 39º C and 5% CO2 with humidified atmosphere. Cleavage was assessed 48 hours after insemination and embryos were morphologically evaluated on day 7 and 8 post insemination (pi) and embryos were morphologically evaluated on day 7 and 8 pi. 4. Statistical analysis. All data are presented as means ± standard deviations. The mean values were compared by using the ANOVA and LSD, and P<0.05 was considered significant. Results Besnoitia besnoiti diagnosis Three bulls, 2 Limousine and 1 Charolais, presented B. besnoiti cysts with live bradoyzoites in the skin (figure 1). Semen evaluation Before cryopreservation, there were no differences (P>0.05) in morphological evaluation (table 1) of semen from asymptomatic infected with Benoitia besnoiti and uninfected bulls. Table 1. Evaluation (mean±SD) asymptomatic infected with Benoitia n=5) before cryopreservation. Volume Gross Bulls (mL) motility control 5.2 ± 2.4 3.0±0.7 INF 6.7 ± 2.8 3.3±0.6 of semen collected by electroejaculation to besnoiti (INF, n=3) and uninfected bulls (control, Individual Concentration motility (%) (105spz mL-1) 82.0±11.0 10.6 ± 3.7 76.7±11.7 8.4 ± 3.6 Dead spz Morphological (%) abnormalities (%) 23.6±13.7 23.4±3.2 16.0±1.7 25.0±1.0 After cryopreservation, there were no significant differences between results from the 2 uninfected bulls running simultaneously in both experiments. Consequently, postthawing results from experiment 1 and 2 were treated together (table 2). From all the sperm quality parameters evaluated, only post-thawed (P=0.05) and post-swim-up (P=0,009) motility were significantly different between asymptomatic infected and uninfected bulls, respectively. Cleavage and embryos rates obtained with semen from uninfected and asymptomatic infected bulls presented no differences (P>0.05). Table 2. Evaluation (mean±SD) of post-thawed semen collected to asymptomatic infected with Benoitia Besnoiti (INF) and uninfected (control) bulls (Host25=hypoosmotic swelling test, 25 minutes). Bulls control INF Post-thawed Motility n Host25 Post-swim-up tests n n Motility Activity Agglutinat. Concentrat (%) (%) (%) (106spzmL-1) a a 45 43.3±10.6 10 54.1±9.6 45 25.1±12.0 2.4±0.7 28.4±12.0 83.6±82.9 b b 15 51.0±18.6 6 45.4±19.1 15 36.3±18.9 2.6±1.1 27.2±10.3 100.5±63.8 Discussion Besnoitia Besnoiti infections are economically important to cattle owners in endemic areas due to mortality, loss of general health condition, low market value and sterility which may be temporary or permanent (Bigalke, 1968, Kumi-Diaka et al., 1981). Prevalence of bovine besnoitiosis in Portugal, especially the asymptomatic form, is not yet clearly determined but may be increasing. In the present study live bradyzoites within Besnoitia cyst were identified although all the bulls tested were asymptomatic. Previous studies (Cortes et al., 2003, 2005) in farms located in Évora region diagnosed 42% of B. Besnoiti infected Limousine bulls without clinical signs of the disease. Asymptomatic B. besnoiti infected bulls presence in herds implies a potential transmission of besnoitiosis although as demonstrated sire fertilitlity is not affected. Infertility or sterility seems to be a late consequence of B. besnoiti infection (Sekoni et al., 1992). Semen quality pre- and post-cryopreservation was not diminished in infected asymptomatic bulls. Neither single parameters of semen quality (motility, sperm number and morphology) having moderate value as predictors of absolute fertility levels (Parkinson, 2004), nor supravital stain and Host tests which can be considered important variables for normal sperm function (Tartaglione & Ritta, 2004) were affected. Also, IVF and embryo rates presented no differences between asymptomatic infected bulls and control. IVF and embryo rates are a useful indicator of bull fertility, as the majority of reports correlated its results with both non-return rates with cryopreserved semen (Marquant-LeGuienne et al., 1990, Ward et al., 2001) and conception rates in natural service (Brahmkshtri et al., 1999). These results suggest that in herds carrying the parasite for a long period, there are no implications on the fertility of subclinically infected bulls. Notwithstanding, we strongly disagree with the transport of infected animals, even if subclinically, to other areas or herds without any contact with this parasite. In fact, due to the mechanical transmission of B. besnoiti by blood-sucking insects or iatrogenicaly from infected to susceptible cattle (Bigalke, 1968), due to the high number of tabanids and Stomoxis calcitrans during summer season, moving infected animals to Besnoitia free herds will contribute to the disease dissemination. Figure 1. Hematoxilin Eosin Stain of a skin, where large number of cysts containing bradizoites are present. Figure 2. Clinical besnoitiosis with thickening, wrinkling and hair loss of the skin. References Aranda Correia, I. (1993). Detecção de cios e inseminação artificial em bovinos. Trabalho de fim de Curso de Produção Animal. Escola Superior Agrária, Instituto Politécnico de Coimbra, 75-76. Thesis in portuguease Basson, P. A., McCully, R. M., & Bigalke, R. D. (1970). Observations on the pathogenesis of bovine and antelope strains of Besnoitia besnoiti (Marotel, 1912) infection in cattle and rabbits. Onderstepoort J. Vet. Res. 37, 105-126. Besnoit, C. & Robin, V. (1912). Sarcosporidioses cutanée chez une vache. Rec. Vet. 37, 649. Bigalke, R. D. (1993). Current Veterinary Therapy 3, Food Animal Practice edited by J. L. Howards, pp. 596-599. W.B. Saunders Company, Philadelphia. Bigalke, R. D. & J. H. Schoeman,1967. An outbreak of bovine besnoitiosis in the orange free state, Republic of South Africa. J. S. A. V. M. A. 38: 435-437. Bigalke, R.D., 1968. New concepts on the epidemiological features of bovine besnoitiosis as determined by laboratory and field investigations. Onderstepoort J. Vet. Res. 35: 3-138. Bourdeau, P. J., Cesbron, N., Alexandre, F., Marchand, A. M., Desvaux, J. P., & Douart, A. (2004). Outbreak of bovine besnoitiosis (Besnoitia besnoiti) in the west of France and its diagnosis by immunofluorescence assay. IX European Multicolloquium of Parasitology, 18-23 July, Valencia, Spain 459-460. Brahmkshtri, B.P., M.J. Edwin, M.C. Jhon, A.M. Nainar, A.M. Krishnan, 1999. Relative efficacy of conventional sperm parameters and sperm penetration bioassay to assess bull fertility in vitro. Anim. Reprod. Scie. 54:159-169. Cortes, H., Ferreira, M. L., Silva, J. F., Vidal, R., Serra, P., & Caeiro, V. (2003). Contribuição para o estudo da besnoitiose bovina em Portugal. Revista Portuguesa de Ciências Veterinárias 98, nº 545, 43-46. Cortes, H., Leitão, A., Vidal, R., Vila-Viçosa, M. J., Ferreira, M. L., Caeiro, V., & Hjerpe, C. A. (2005). Bovine Besnoitiosis in Portugal. (in press) Ferreira, G.M., J.P. Sousa, J.P. Barbas & A.E.M. Horta, 2001. Buck semen Hos test of Serrana breed. 3rd Iberian Congress on Animal Reproduction, Porto. pp.559-564. Portuguese Animal Reproduction Society (ed.) Ferreira, M. L., Nunes Petisca, J. L., & Dias, O. H. (1982). Alterações testiculares em touros de Moçambique assintomáticos e com sintomas clínicos de besnoitiose. Rep. Trab. I. N. V. XIV, 97-108. Franco, E. E. & Borges, I. (1915). Nota sobre a sarcosporidiose bovina. Revista de Medicina Veterinária Ano XIV, 255-299. Irigoien, M., Del Cacho, E., Gallego, M., Lopez-Bernad, F., Quilez, J., & SanchezAcedo, C. (2000). Immunohistochemical study of the cyst of Besnoitia besnoiti. Vet. Parasitol. 91, 1-6. Juste, R. A., Cuervo, L. A., Marco, J. C., & Oregui, L. M. (1990). La besnoitiosis bovina: desconocida en España? Medicina Veterinária 7, 613-618. Kumi-Diaka, J., Wilson, S., Sannusi, A., Njoku, C. E., & Osoru, D. I. K. (1981). Bovine besnoitiosis and its effect on the male reproductive system. Theriogenology 16, 523530. Leitão, J. L. S. (1949). Globidiose bovina por Globidium besnoiti (Marotel 1912). Revista de Medicina Veterinária 330, 152-158. Malta, M. & Silva, M. (1991). Besnoitiose no Alentejo. XIV Jornadas de MEdicina Veterinária "Bovinos de Carne". Personal communication. Marquant-LeGuienne, B., P. Humblot, M. Thibier, C. Thibault, 1990. Evaluation of bull semen fertility by homologous in vitro fertilization tests. Rep. Nutr. Dev. 30: 259-266. McCully, R. M., P.A. Basson, J.W. van Niekerk & R.D. Bigalke, 1966. Observations on Besnoitia cysts in the cardiovascular system of some wild antelopes and domestic cattle. Onderstepoort J. Vet. Res. 33, 245-276. Parkinson, T.J., 2004. Evaluation of fertility and infertility in natural service bulls. Vet. J. 168: 215-229. Pereira R.M., C.C. Marques, M.C. Baptista, M.I. Vasques & A.E.M. Horta, 2005. Influence of supplementation of arachidonic acid and cyclooxygenase/lipoxygenase inhibition on the development of early bovine embryos. Rev. Braz. Zoot. (accepted). Pols, J. W. (1960). Studies on Bovine besnoitiosis with special reference to the aetiology. Onderstepoort J. Vet. Res. 28, 265-356. Sekoni, V.O., A. Sanusi, M.O. Abata, E.O. Oyedipe, P.I. Rekwot & L.O. Eduvie, 1992. Loss of libido and terminal sterility in a Frisean bull naturally infected with Benoitia besnoiti in Northern Nigeria: A case report. Theriogenology 37: 533-549. Shkap, V., Pipano, E., Marcus, S., & Krigel, Y. (1994). Bovine besnoitiosis: transfer of colostral antibodies with observations possibly relating to natural transmission of the infection. Onderstepoort J. Vet. Res. 61, 273-275. Tartaglione, C.M. & M.N. Ritta, 2004. Prognostic value of spermatological parameters as predictors of invitro fertility of frozen-thawed bull semen. Theriogenology 62: 12451252. Ward, F., D. Rizos, D. Corridan, K. Quinn, M. Boland & P. Lonergan, 2001. Paternal influence on the time of firsat embryonic cleavage pot insemination and the implications for subsequent bovine embryo development in vitro and fertility in vivo. Mol. Reprod. Dev. 60: 47-55.